People are exposed to contaminants through the respiratory tract and skin; they first reach the bloodstream and, subsequently, the organs, causing more or less serious damage to health. Thus, the effects of atmospheric pollution affect the respiratory tract with acute symptoms and the circulatory system with cardiovascular events, leading to hospitalizations and mortality. In addition to the acute effects, long-term effects can also be had, including an alteration of lung function in adults, children, and adolescents. Specifically, in children and adolescents, chronic exposure to air pollution is associated with a reduction in forced vital capacity (FVC), which correlates with age and can be interpreted as a reduction in the lung growth and respiratory function of the lower airways. Children, together with elderly persons, are the most sensitive subjects to environmental pollution; to these are added subjects with chronic respiratory diseases such as asthma and chronic obstructive pulmonary disease (COPD). Emerging contaminants induce pulmonary toxicity by promoting an inflammatory response in lung epithelial cells.
- environmental pollution
- airway diseases
- epithelial cells
- system biology
1. Introduction
2. Cell Systems to Study the Effects of Environmental Contaminants in Respiratory Diseases
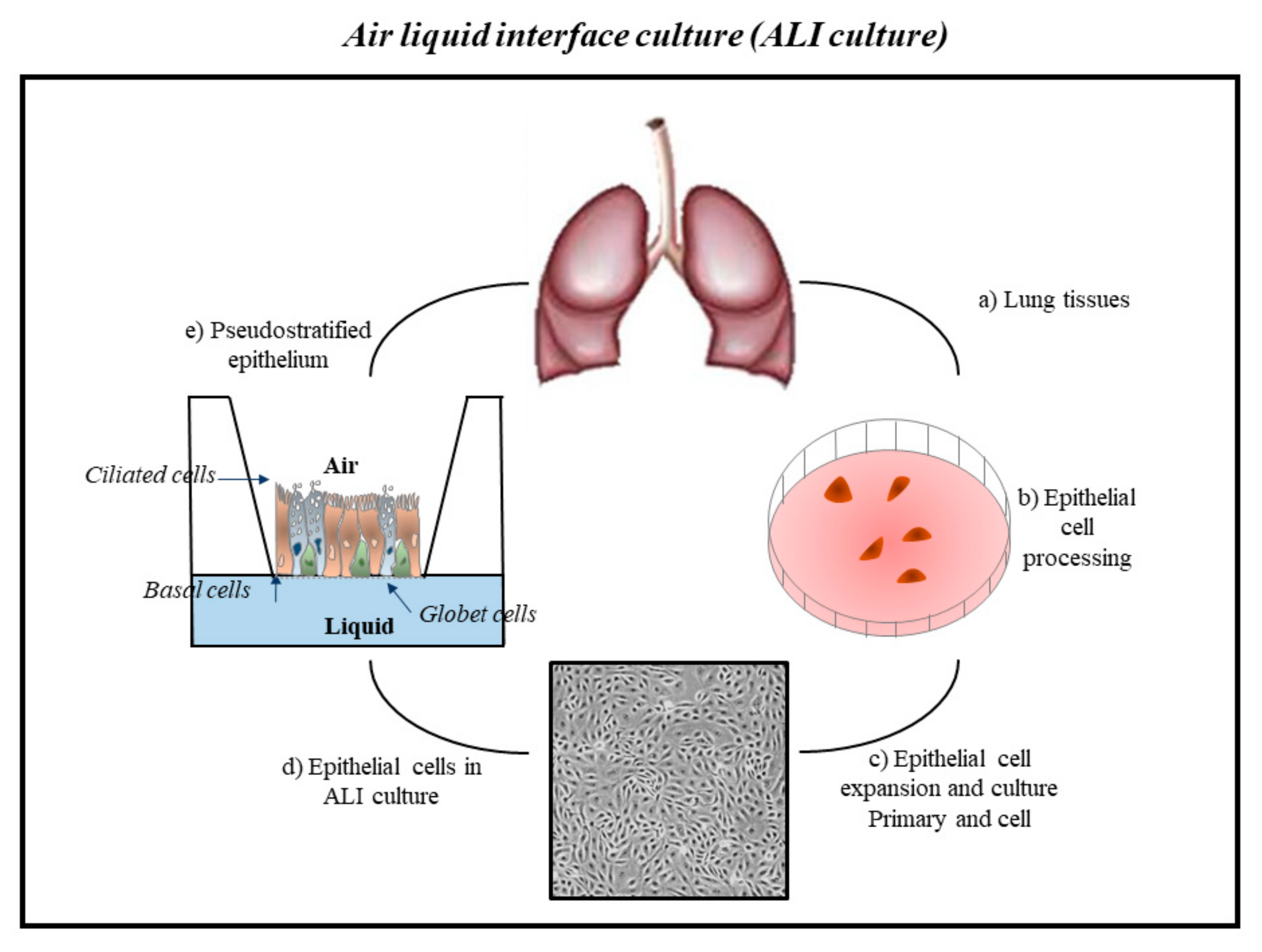

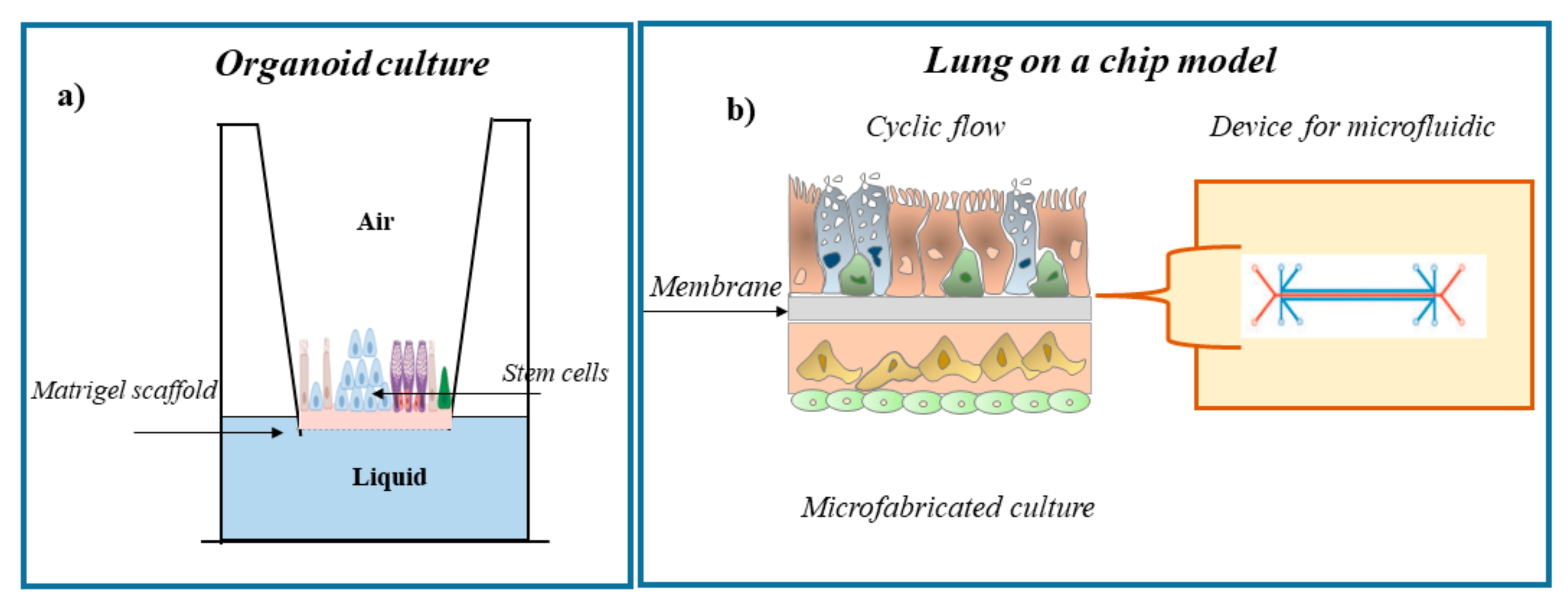
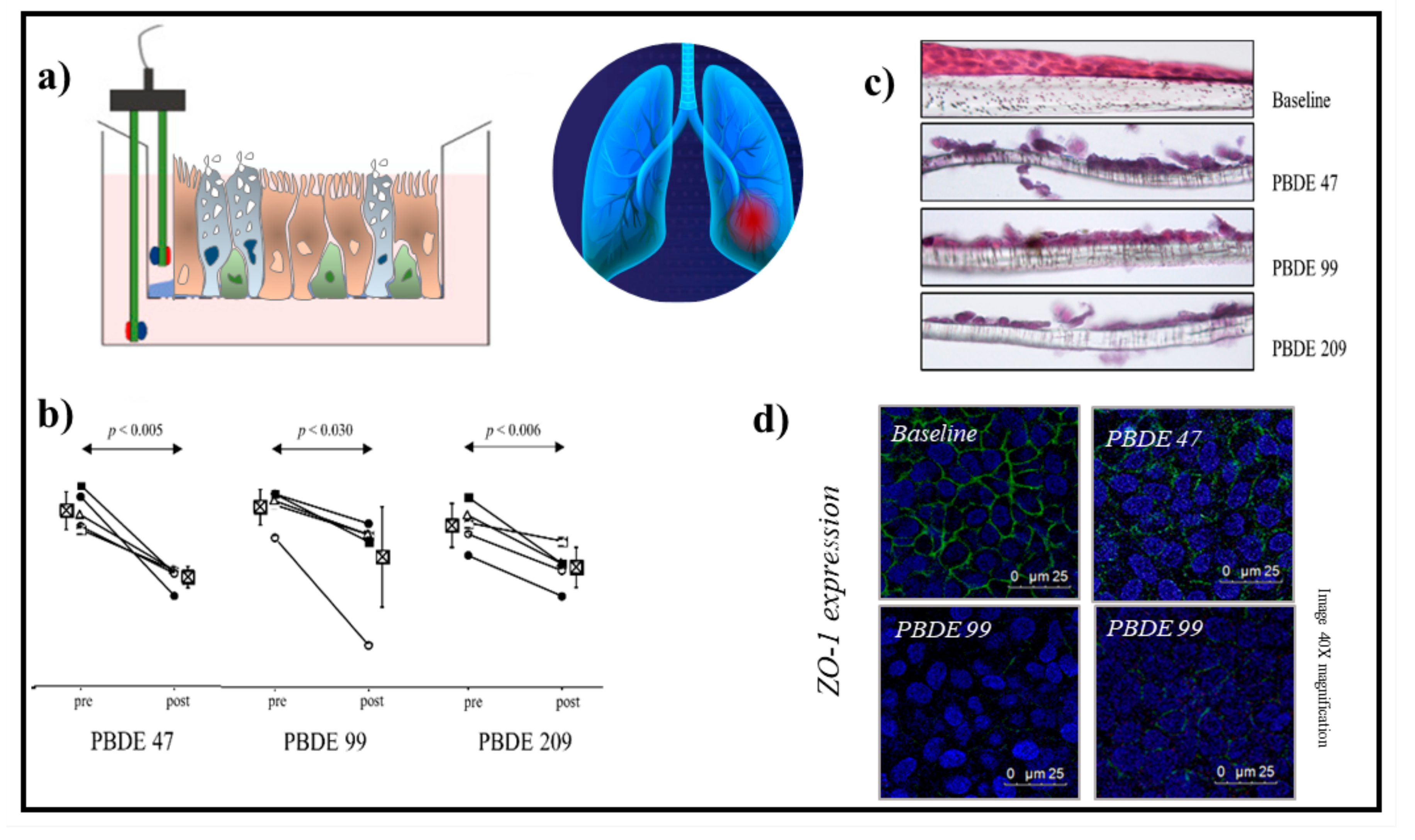
3. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/ijms23052799
References
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and health im-pacts of air pollution: A review. Front. Public Health. 2020, 20, 8–14.
- Ashfaq, A.; Sharma, P. Environmental effects of air pollution and application of engineered methods to combat the problem. J. Indust. Pollut. Control. 2012, 29, 25–28.
- Gauderman, W.J.; Avol, E.; Gilliland, F.; Vora, H.; Thomas, D.; Berhane, K.; McConnell, R.; Kuenzli, N.; Lurmann, F.; Rappaport, E.; et al. The effect of air pollution on lung development from 10 to 18 years of age. N. Engl. J. Med. 2004, 351, 1057–1067, Erratum in 2005, 352, 1276.
- Inquinamento e Salute. Dal Traffico al Fumo, Dalla Chimica all’attività Lavorativa: Come l’ambiente Influenza il Rischio di Ammalarsi di Tumore. Fondazioneveronesi.it. 2018. Available online: https://www.fondazioneveronesi.it/magazine/tools-della-salute/download/i-manuali/inquinamento-e-salute (accessed on 24 January 2022).
- Proietti, M. Inquinamento e Malattie. Edizioni Minerva Medica 2018. Available online: https://www.minervamedica.it/it/volumi/specialitadiche/igiene/scheda.php?cod=L10091 (accessed on 24 January 2022).
- Berend, N. Contribution of air pollution to COPD and small airway dysfunction. Respirology. 2016, 21, 237–244.
- Holland, W.W.; Reid, D.D. The urban factor in chronic bronchitis. Lancet 1965, 1, 445–448.
- Schwartz, J. Lung function and chronic exposure to air pollution: A cross-sectional analysis of NHANES II. Environ. Res. 1989, 50, 309–321.
- Robertson, D.S. Health effects of increase in concentration of carbon dioxide in the atmosphere. Curr. Sci. 2006, 90, 1607–1609.
- Zhao, Y.; Hu, J.; Tan, Z.; Liu, T.; Zeng, W.; Li, X.; Huang, C.; Wang, S.; Huang, Z.; Ma, W. Ambient carbon monoxide and increased risk of daily hospital out-patient visits for respiratory diseases in Dongguan, China. Sci. Total Environ. 2019, 668, 254–260.
- Hamra, G.B.; Laden, F.; Cohen, A.J.; Raaschou-Nielsen, O.; Brauer, M.; Loomis, D. Lung cancer and expo-sure to nitrogen dioxide and traffic: A systematic review and meta-analysis. Environ. Health Perspect. 2015, 123, 1107–1112.
- Faustini, A.; Rapp, R.; Forastiere, F. Nitrogen dioxide and mortality: Review andmeta-analysis of long-termstudies. Eur. Respir. J. 2014, 44, 744–753.
- Nriagu, J.O. Air pollution from solid fuels. In Encyclopedia of Environmental Health; Elsevier: Amsterdam, The Netherlands, 2011; pp. 46–52.
- Abdel-Shafy, H.I.; Mansour, M.S.M. A review on polycyclic aromatic hydrocarbons: Source, envi-ronmental impact, effect on human health and remediation. Egypt J. Pet. 2016, 25, 107–123.
- Arpalombardia. 2018. Available online: https://www.arpalombardia.it/Pages/Aria/Inquinanti/Metalli.aspx?firstlevel=Inquinanti (accessed on 24 January 2022).
- Brunekreef, B.; Beelen, R.; Hoek, G.; Schouten, L.; Bausch-Goldbohm, S.; Fischer, P.; Armstrong, B.; Hughes, E.; Jerrett, M.; van den Brandt, P. Effects of long-term exposure to traffic-related air pol-lution on respiratory and cardiovascular mortality in the Netherlands: The NLCS-AIR study. Res. Rep. 2009, 139, 5–71.
- Colais, P.; Faustini, A.; Stafoggia, M.; Berti, G.; Bisanti, L.; Cadum, E.; Cernigliaro, A.; Mallone, S.; Pacelli, B.; Serinelli, M.; et al. EPIAIR Collaborative Group. Particulate air pollution and hospital admissions for cardiac diseases in potentially sensitive subgroups. Epidemiology 2012, 23, 473–481.
- Beelen, R.; Hoek, G.; van den Brandt, P.A.; Goldbohm, R.A.; Fischer, P.; Schouten, L.J.; Armstrong, B.; Brunekreef, B. Long-term exposure to traffic-related air pollution and lung cancer risk. Epidemiology 2008, 19, 702–710.
- Vineis, P.; Hoek, G.; Krzyzanowski, M.; Vigna-Taglianti, F.; Veglia, F.; Airoldi, L.; Autrup, H.; Dunning, A.; Garte, S.; Hainaut, P.; et al. Air pollution and risk of lung cancer in a prospective study in Europe. Int. J. Cancer 2006, 119, 169–174.
- Palli, D.; Saieva, C.; Munnia, A.; Peluso, M.; Grechi, D.; Zanna, I.; Caini, S.; Decarli, A.; Sera, F.; Masala, G. DNA adducts and PM10 exposure in traffic-exposed workers and urban residents from the EPIC-Florence city study. Sci. Total Environ. 2008, 403, 105–112.
- Heinrich, J.; Thiering, E.; Rzehak, P.; Krämer, U.; Hochadel, M.; Rauchfuss, K.M.; Gehring, U.; Wichmann, H.E. Long-term exposure to NO2 and PM10 and all-cause and cause-specific mortality in a prospective cohort of women. Occup. Environ. Med. 2013, 70, 179–186.
- Raaschou-Nielsen, O.; Andersen, Z.J.; Beelen, R.; Samoli, E.; Stafoggia, M.; Weinmayr, G.; Hoffmann, B.; Fischer, P.; Nieuwenhuijsen, M.J.; Brunekreef, B.; et al. Air pollution and lung cancer incidence in 17 european cohorts: Prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE). Lancet Oncol. 2013, 14, 813–822.
- Schmid, O.; Stoeger, T. Surface area is the biologically most effective dose metric for acute nanoparticle toxicity in the lung. J. Aerosol. Sci. 2016, 99, 133–143.
- Teeguarden, J.G.; Hinderliter, P.M.; Orr, G.; Thrall, B.D.; Pounds, J.G. Particokinetics in vitro: Dosimetry considerations for in vitro nanoparticle toxicity assessments. Toxicol. Sci. 2007, 95, 300–312, Erratum in Toxicol. Sci. 2007, 97, 614.
- Wilkinson, K.E.; Palmberg, L.; Witasp, E.; Kupczyk, M.; Feliu, N.; Gerde, P.; Seisenbaeva, G.A.; Fadeel, B.; Dahlen, S.E.; Kessler, V.G. Solution engineered palladium nanoparticles: Model for health effect studies of automotive particulate pollution. ACS Nano 2011, 5, 5312–5324.
- International Agency for Research on Cancer (IARC). Air Pollution and Cancer; Straif, K., Cohen, A., Samet, J., Eds.; IARC Scientific Publication: Lyon, France, 2013; ISBN1 13978-92-832-2166-1. ISBN2 13978-92-832-2161-6.
- Piscitelli, P.; Valenzano, B.; Rizzo, E.; Maggiotto, G.; Rivezzi, M.; Esposito Corcione, F.; Miani, A. Air pollution and estimated health costs related to road transportations of goods in Italy: A first healthcare burden assessment. Int. J. Environ. Res. Public Health 2019, 16, 2876.
- Air Pollution—European Environment Agency. 2021. Available online: https://www.eea.europa.eu/themes/air/intro (accessed on 24 January 2022).
- GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir. Med. 2017, 5, 691–706, Erratum in Lancet Respir. Med. 2017, 5, e30.
- International Agency for Research on Cancer (IARC). Outdoor Air Pollution 2016—Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer (IARC): Lyon, France, 2016; Volume 109, ISBN1 13-978-92-832-0147-2. ISBN2 13-978-92-832-0175-5.
- Martin, P.J.; Héliot, A.; Tremolet, G.; Landkocz, Y.; Dewaele, D.; Cazier, F.; Ledoux, F.; Courcot, D. Cellular response and extracellular vesicles characteri-zation of human macrophages exposed to fine atmospheric particulate matter. Environ. Pollut. 2019, 254 Pt A, 112933.
- Guarnieri, M.; Balmes, J.R. Outdoor air pollution and asthma. Lancet 2014, 383, 1581–1592.
- Galasso, R.; Gruppo di lavoro Sentieri. SENTIERI/Quinto Rapporto—Studio Epidemiologico Na-zionale dei Territori e degli Insediamenti Esposti a Rischio da Inquinamento. Valutazione della evidenza epidemiologica. Epidemiol. Prev. 2019, 43 (Suppl. 2–3), 1–208.
- Ko, F.W.S.; Hui, D.S.C. Effects of air pollution on lung health. Clin. Pulm. Med. 2010, 17, 300–304.
- Pirastu, R.; Ancona, A.; Iavarone, I.; Mitis, F.; Zona, A.; Comba, P. SENTIERI/Quinto Rapporto—Studio Epidemiologico Nazionale dei Territori e degli Insediamenti Esposti a Rischio da Inqui-namento. Valutazione della evidenza epidemiologica. Epidemiol. Prev. 2010, 34 (Suppl. 3), 1–96.
- Degrendele, C.; Wilson, J.; Kukučka, P.; Klánová, J.; Lemmel, G. Are atmospheric PBDE levels declin-ing in central Europe? Examination of the seasonal and semi-long-term variations, gas—particle partitioning and implications for long-range atmospheric transport. Atmos. Chem. Phys. 2018, 18, 12877–12890.
- Besis, A.; Lammel, G.; Kukučka, P.; Samara, C.; Sofuoglu, A.; Dumanoglu, Y.; Eleftheriadis, K.; Kouvarakis, G.; Sofuoglu, S.C.; Vassilatou, V.; et al. Polybrominated diphenyl ethers (PBDEs) in background air around the Aegean: Implications for phase partitioning and size distribution. Environ. Sci. Pollut. Res. Int. 2017, 24, 28102–28120.
- Yuan, Y.; Meeker, J.D.; Ferguson, K.K. Serum polybrominated diphenyl ether (PBDE) concentrations in relation to biomarkers of oxidative stress and inflammation: The National Health and Nutrition Examination Survey 2003–2004. Sci. Total Environ. 2017, 575, 400–405.
- Kim, J.S.; Klösener, J.; Flor, S.; Peters, T.M.; Ludewig, G.; Thorne, P.S.; Robertson, L.W.; Luthe, G. Toxicity assessment of air-delivered particle-bound polybro-minated diphenyl ethers. Toxicology 2014, 317, 31–39.
- Montalbano, A.M.; Albano, G.D.; Anzalone, G.; Moscato, M.; Gagliardo, R.; Di Sano, C.; Bonanno, A.; Ruggieri, S.; Cibella, F.; Profita, M. Cytotoxic and genotoxic effects of the flame retardants (PBDE-47, PBDE-99 and PBDE-209) in human bronchial epithelial cells. Chemosphere 2020, 245, 125600.
- Pozo, K.; Palmeri, M.; Palmeri, V.; Estellano, V.H.; Mulder, M.D.; Efstathiou, C.I.; Sará, G.L.; Romeo, T.; Lammel, G.; Focardi, S. Assessing persistent organic pollutants (POPs) in the Sicily island atmosphere, mediterranean, using PUF disk passive air samplers. Environ. Sci. Pollut. Res. Int. 2016, 23, 20796–20804.
- Santoro, A.; Ferrante, M.C.; Di Guida, F.; Pirozzi, C.; Lama, A.; Simeoli, R.; Clausi, M.T.; Monnolo, A.; Mollica, M.P.; Mattace Raso, G.; et al. Polychlorinated Biphenyls (PCB 101, 153, and 180) Impair Murine Macrophage Responsiveness to Lipopolysaccharide: Involvement of NF-κB Pathway. Toxicol Sci. 2015, 147, 255–269.
- Frederiksen, M.; Vorkamp, K.; Mathiesen, L.; Mose, T.; Knudsen, L.E. Placental transfer of the polybrominated di-phenyl ethers BDE-47, BDE-99 and BDE-209 in a human placenta perfusion system: An exper-imental study. Environ. Health 2010, 9, 32.
- La Guardia, M.J.; Hale, R.C.; Harvey, E. Detailed polybrominated diphenyl ether (PBDE) congener composition of the widely used penta-, octa-, and deca-PBDE technical flame-retardant mix-tures. Environ. Sci. Technol. 2006, 40, 6247–6254.
- ECD. Official Journal of the European Union Commission Decision 2005/717/EC—Exemption of DecaBDE from the Prohibition on Use, C 116 (9 May 2008). 2009. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:C:2008:116:FULL&from=PL (accessed on 24 January 2022).
- Crystal, R.G.; Randell, S.H.; Engelhardt, J.F.; Voynow, J.; Sunday, M.E. Airway epithelial cells: Current concepts and challenges. Proc. Am Thorac. Soc. 2008, 5, 772–777.
- Schechtman, L.M. Implementation of the 3Rs (refinement, reduction, and replacement): Vali-dation and regulatory acceptance considerations for alternative toxicological test methods. ILAR J. 2002, 43, S85–S94.
- Belliveau, M.E. The drive for a safer chemicals policy in the United States. New Solut. 2011, 21, 359–386.
- Impinen, A.; Nygaard, U.C.; Carlsen, K.L.; Mowinckel, P.; Carlsen, K.H.; Haug, L.S.; Granum, B. Prenatal exposure to perfluoralkyl sub-stances (PFASs) associated with respiratory tract infections but not allergy- and asthma-related health outcomes in childhood. Environ. Res. 2018, 160, 518–523.
- Tannenbaum, J.; Bennett, B.T. Russell and Burch’s 3Rs then and now: The need for clarity in definition and purpose. J. Am. Assoc. Lab. Anim Sci. 2015, 54, 120–132.
- Eagle, H. Nutrition needs of mammalian cells in tissue culture. Science 1955, 122, 501–514.
- Eagle, H. The specific amino acid requirements of a human carcinoma cell (Stain HeLa) in tis-sue culture. J. Exp. Med. 1955, 102, 3748.
- Ritter, D.; Knebel, J.; Niehof, M.; Loinaz, I.; Marradi, M.; Gracia, R.; Te Welscher, Y.; van Nostrum, C.F.; Falciani, C.; Pini, A.; et al. In vitro inhalation cytotoxicity testing of therapeutic nanosystems for pulmonary infection. Toxicol. In Vitro 2020, 63, 104714.
- BeruBe, K.; Aufderheide, M.; Breheny, D.; Clothier, R.; Combes, R.; Duffin, R.; Forbes, B.; Gaca, M.; Gray, A.; Hall, I.; et al. In vitro models of inhalation toxicity and disease. The report of a FRAME workshop. Altern. Lab. Anim. 2009, 37, 89–141.
- Hiemstra, P.S.; Grootaers, G.; van der Does, A.M.; Krul, C.A.M.; Kooter, I.M. Human lung epithelial cell cultures for analysis of inhaled toxicants: Lessons learned and future directions. Toxicol. In Vitro 2018, 47, 137–146.
- Lewinski, N.A.; Liu, N.J.; Asimakopoulou, A.; Papaioannou, E.; Konstandopoulos, A.; Riediker, M. Air-liquid interface cell exposures to nanoparticle aerosols. Methods Mol. Biol. 2017, 1570, 301–313.
- Polk, W.W.; Sharma, M.; Sayes, C.M.; Hotchkiss, J.A.; Clippinger, A.J. Aerosol generation and charac-terization of multi-walled carbon nanotubes exposed to cells cultured at the airliquid interface. Part. Fibre Toxicol. 2016, 13, 20.
- Kim, J.S.; Peters, T.M.; O’Shaughnessy, P.T.; Adamcakova-Dodd, A.; Thorne, P.S. Validation of an in vitro exposure system for toxicity assessment of air-delivered nanomaterials. Toxicol. In Vitro 2013, 27, 164–173.
- Ji, J.; Hedelin, A.; Malmlöf, M.; Kessler, V.; Seisenbaeva, G.; Gerde, P.; Palmberg, L. Development of combining of human bronchial mucosa models with XposeALI(R) for exposure of air pollution nanoparticles. PLoS ONE 2017, 12, e0170428.
- Dvorak, A.; Tilley, A.E.; Shaykhiev, R.; Wang, R.; Crystal, R.G. Do airway epithelium air-liquid cultures represent the in vivo airway epithelium transcriptome? Am. J. Respir. Cell Mol. Biol. 2011, 44, 465–473.
- Rock, J.R.; Onaitis, M.W.; Rawlins, E.L.; Lu, Y.; Clark, C.P.; Xue, Y.; Randell, S.H.; Hogan, B.L. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl. Acad. Sci. USA 2009, 106, 12771–12775.
- Tadokoro, T.; Wang, Y.; Barak, L.S.; Bai, Y.; Randell, S.H.; Hogan, B.L. IL-6/STAT3 promotes regenera-tion of airway ciliated cells from basal stem cells. Proc. Natl. Acad. Sci. USA 2014, 111, E3641–E3649.
- Polosukhin, V.V.; Cates, J.M.; Lawson, W.E.; Milstone, A.P.; Matafonov, A.G.; Massion, P.P.; Lee, J.W.; Randell, S.H.; Blackwell, T.S. Hypoxiainducible factor-1 signalling promotes goblet cell hyperplasia in airway epithelium. J. Pathol. 2011, 224, 203–211.
- Gao, X.; Bali, A.S.; Randell, S.H.; Hogan, B.L. GRHL2 coordinates regeneration of a polarized mucocil-iary epithelium from basal stem cells. J. Cell Biol. 2015, 211, 669–682.
- Upadhyay, S.; Palmberg, L. Air-Liquid Interface: Relevant in vitro models for investigating air pollutant-induced pulmonary toxicity. Toxicol. Sci. 2018, 164, 21–30.
- King, P.T. Inflammation in chronic obstructive pulmonary disease and its role in cardiovascular disease and lung cancer. Clin. Transl. Med. 2015, 4, 68.
- Faber, S.C.; McCullough, S.D. Through the looking glass: In vitro models for inhalation toxicology and interindividual variability in the airway. Appl. In Vitro Toxicol. 2018, 4, 115–128.
- Gordon, S.; Daneshian, M.; Bouwstra, J.; Caloni, F.; Constant, S.; Davies, D.E.; Dandekar, G.; Guzman, C.A.; Fabian, E.; Haltner, E.; et al. Non-animal models of epithelial barriers (skin, in-testine and lung) in research, industrial applications and regulatory toxicology. Altex 2015, 32, 327–378.
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a dish: Modeling development and disease using organoid technologies. Scence 2014, 345, 1247125.
- Fatehullah, A.; Tan, S.H.; Barker, N. Organoids as an in vitro model of human development and disease. Nat. Cell Biol. 2016, 18, 246–254.
- Pohl, C.; Hofmann, H.; Moisch, M.; Papritz, M.; Hermanns, M.I.; Dei-Anang, J.; Mayer, E.; Kehe, K.; Kirkpatrick, C.J. Acute cytotoxicity and apoptotic effects after l-Pam ex-posure in different cocultures of the proximal and distal respiratory system. J. Biotechnol. 2010, 148, 31–37.
- Papritz, M.; Pohl, C.; Wübbeke, C.; Moisch, M.; Hofmann, H.; Hermanns, M.I.; Thiermann, H.; Kirkpatrick, C.J.; Kehe, K. Side-specific effects by cadmium exposure: Apical and basolateral treatment in a coculture model of the blood-air barrier. Toxicol. Appl. Pharmacol. 2010, 245, 361–369.
- Emmler, J.; Hermanns, M.I.; Steinritz, D.; Kreppel, H.; Kirkpatrick, C.J.; Bloch, W.; Szinicz, L.; Kehe, K. Assessment of alterations in barrier functionality and induction of proinflammatory and cytotoxic effects after sulfur mustard exposure of an in vitro coculture model of the human alveolo-capillary barrier. Inhal. Toxicol. 2007, 19, 657–665.
- Miller, A.J.; Hill, D.R.; Nagy, M.S.; Aoki, Y.; Dye, B.R.; Chin, A.M.; Huang, S.; Zhu, F.; White, E.S.; Lama, V.; et al. In vitro induction and in vivo engraftment of lung bud tip progenitor cells derived from human pluripotent stem cells. Stem Cell Rep. 2018, 10, 101–119.
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting organ-level lung functions on a chip. Science 2010, 328, 1662–1668.
- Clevers, H. Modeling development and disease with organoids. Cell 2016, 165, 1586–1597.
- Paget, V.; Dekali, S.; Kortulewski, T.; Grall, R.; Gamez, C.; Blazy, K.; Aguerre-Chariol, O.; Chevillard, S.; Braun, A.; Rat, P.; et al. Specific uptake and genotoxicity induced by polystyrene nanobeads with distinct surface chemistry on human lung epithelial cells and macrophages. PLoS ONE 2015, 10, e0123297.
- Nalayanda, D.D.; Wang, Q.; Fulton, W.B.; Wang, T.H.; Abdullah, F. Engineering an artificial alveolar-capillary membrane: A novel continuously perfused model within microchannels. J. Pediatr. Surg. 2010, 45, 45–51.
- Stucki, A.O.; Stucki, J.D.; Hall, S.R.; Felder, M.; Mermoud, Y.; Schmid, R.A.; Geiser, T.; Guenat, O.T. A lung-on-a chip array with an integrated bio-inspired respiration mechanism. Lab. Chip 2015, 15, 1302–1310.
- Huh, D.D. A human breathing lung-on-a-chip. Ann. Am. Thorac. Soc. 2015, 12 (Suppl. 1), S42–S44.
- Fishler, R.; Sznitman, J. A microfluidic model of biomimetically breathing pulmonary acinar airways. J. Vis. Exp. 2016, 9, e53588.
- Cromwell, O.; Hamid, Q.; Corrigan, C.J.; Barkans, J.; Meng, Q.; Collins, P.D.; Kay, A.B. Expression and generation of interleukin-8, IL-6 and granulocyte-macrophage colony-stimulating factor by bronchial epithelial cells and enhance-ment by IL-1 beta and tumour necrosis factor-alpha. Immunology 1992, 77, 330–337.
- Kotas, M.E.; Medzhitov, R. Homeostasis, inflammation, and disease susceptibility. Cell 2015, 160, 816–827.
- Schleimer, R.P.; Kato, A.; Kern, R.; Kuperman, D.; Avila, P.C. Epithelium: At the interface of innate and adaptive immune responses. J. Allergy Clin. Immunol. 2007, 120, 1279–1284.
- Grilli, A.; Bengalli, R.; Longhin, E.; Capasso, L.; Proverbio, M.C.; Forcato, M.; Bicciato, S.; Gualtieri, M.; Battaglia, C.; Camatini, M. Transcriptional profiling of human bronchial epithelial cell BEAS-2B exposed to diesel and biomass ultrafine particles. BMC Genomics 2018, 19, 302.
- McCullough, S.D.; On, D.M.; Bowers, E.C. Using chromatin immunoprecipitation in toxicology: A Step-by-step guide to increasing efficiency, reducing variability, and expanding applications. Curr. Protoc. Toxicol. 2017, 72, 3.14.1–3.14.28.
- Lenz, A.G.; Karg, E.; Brendel, E.; Hinze-Heyn, H.; Maier, K.L.; Eickelberg, O.; Stoeger, T.; Schmid, O. Inflammatory and oxidative stress responses of an alveolar epithelial cell line to airborne zinc oxide nanoparticles at the air-liquid interface: A comparison with conventional, submerged cell-culture conditions. Biomed. Res. Int. 2013, 2013, 652632.
- Salthammer, T.; Bahadir, M. Occurrence, dynamics and reactions of organic pol-lutants in the indoor environment. Clean 2009, 37, 417–435.
- Petry, T.; Vitale, D.; Joachim, F.J.; Smith, B.; Cruse, L.; Mascarenhas, R.; Schneider, S.; Singal, M. Human health risk evaluation of selected VOC, SVOC and particulate emissions from scented candles. Regul. Toxicol. Pharmacol. 2014, 69, 55–70.
- Tsoutsoulopoulos, A.; Möhle, N.; Aufderheide, M.; Schmidt, A.; Thiermann, H.; Steinritz, D. Optimization of the CULTEX(®) radial flow system for in vitro investigation of lung damaging agents. Toxicol. Lett. 2016, 244, 28–34.
- Gminski, R.; Tang, T.; Mersch-Sundermann, V. Cytotoxicity and genotoxicity in human lung epithelial A549 cells caused by airborne volatile organic compounds emitted from pine wood and oriented strand boards. Toxicol. Lett. 2010, 196, 33–41.
- Dwivedi, A.M.; Upadhyay, S.; Johanson, G.; Ernstgård, L.; Palmberg, L. Inflammatory effects of acrolein, crotonaldehyde and hexanal vapors on human primary bronchial epithelial cells cultured at air-liquid interface. Toxicol. In Vitro 2018, 46, 219–228.
- Gostner, J.M.; Zeisler, J.; Alam, M.T.; Gruber, P.; Fuchs, D.; Becker, K.; Neubert, K.; Kleinhappl, M.; Martini, S.; Überall, F. Cellular reactions to long-term volatile organic compound (VOC) exposures. Sci. Rep. 2016, 6, 37842.
- Siroux, V.; Agier, L.; Slama, R. The exposome concept: A challenge and a potential driver for environmental health research. Eur. Respir. Rev. 2016, 25, 124–129.
- Rim, K. In vitro Models for Chemical Toxicity: Review of their applications and prospects. Toxicol. Environ. Health Sci. 2019, 11, 94–103.
