Minerals and trace elements are important micronutrients for normal physiological function of the body. They are abundant in natural food sources and are regularly included in dietary supplements whereas highly processed industrial food often contains reduced or altered amounts of them. In modern society, the daily intake, storage pools, and homeostasis of these micronutrients are dependent on certain dietary habits and can be thrown out of balance by malignancies. Their dietary imbalance, which is becoming more common in the diets of industrialized countries, is linked to an increased risk of cancer.
- cancer
- biomarkers
- minerals
- copper
- zinc
- selenium
- iron
- iodine
- phosphorus
- calcium
1. Introduction
| Mineral | RDA/PRI (µg/Day) |
EAR/AR (µg/Day) |
UL (µg/Day) |
Serum Levels | Source | References |
|---|---|---|---|---|---|---|
| Iron | 8000 | 6000 | 45,000 | 30 µg/L † | Meat, fish, cereals, beans, nuts. | [12][13][14] |
| Zinc | 8000–11,000 | 9400 | 25,000 | ≥800 µg/L ‡ | Meat, legumes, eggs, fish, grains. | [12][15][16] |
| Selenium | 30–70 | 70 | 300 | 47–145 µg/L | Meat, fish. | [12][17] |
| Phosphorus | 700,000 | 580,000 | n.d. | 0.8–1.5 × 103 µmol/L | Meat, fish. | [12][18] |
| Calcium | 1,000,000 | 750,000 | 2,500,000 | 2500 µmol/L | Milk, fish, legumes. | [12][14][19] |
| Copper | 900 | 1600 | 5000 | 1200 µg/L | Milk, fish, eggs, vegetables. | [12][20] |
| Iodine | 150 | 95 | 600 | 40–80 µg/L | Marine products, eggs, milk, iodized salt. | [12][21] |
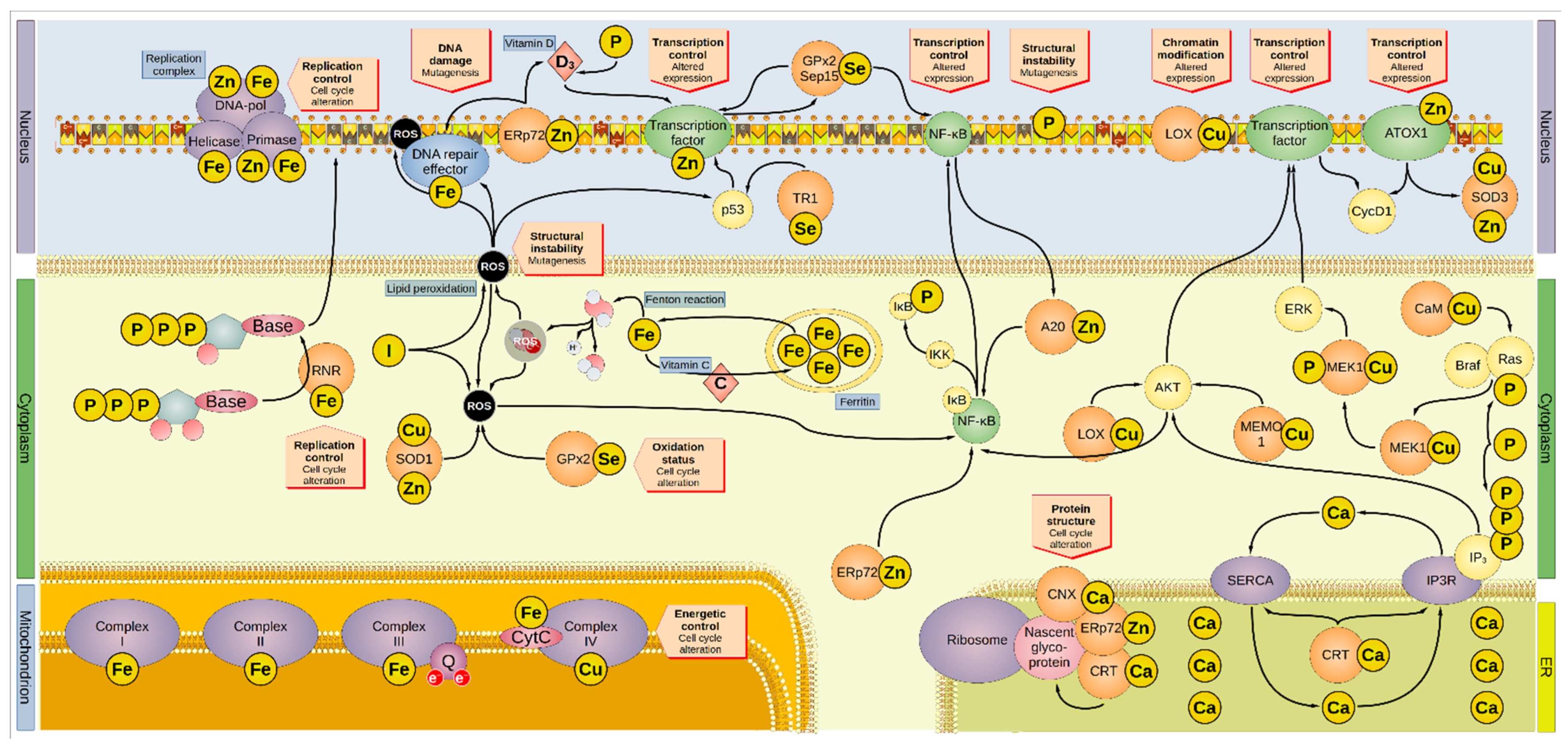
2. Minerals and Risk of Cancer
| Mineral | Organ | Sample | Association * | Measure † | Reference |
|---|---|---|---|---|---|
| Zinc | Breast | Tissue | Direct | Qt | [22] |
| Brain | Tissue | Inverse | Qt | [23] | |
| Intake | Inverse | Qt | [24] | ||
| Mouth | Intake | None | Qt | [25] | |
| Serum | Inverse | Qt | [26] | ||
| Liver | Serum | None | HR | [27] | |
| Serum | Direct | Qt | [28] | ||
| Colon | Tissue | Inverse | OR | [29] | |
| Copper | Liver | Serum | Direct | HR | [27] |
| Serum | Direct | Qt | [28] | ||
| Mouth | Serum | Direct | Qt | [26] | |
| Colon | Tissue | Direct | OR | [29] | |
| Brain | Tissue | Inverse | Qt | [23] | |
| Serum | Direct | Qt | [30] | ||
| Intake | Inverse | Qt | [24] | ||
| Pancreas | Serum | Direct | Qt | [31] | |
| Selenium | Esophagus | Tissue | Direct | Qt | [32] |
| Prostate | Tissue | None | Qt | [33] | |
| Serum | None ║ | Qt | [34] | ||
| Any | Serum | None | Qt | [35] | |
| Serum | Inverse | Qt | [36] | ||
| Liver | Serum | Inverse | Qt | [37] | |
| Serum | Inverse | Qt | [38] | ||
| Colon | Serum | None ¶ | IR | [39] | |
| Pancreas | Serum | Inverse | OR | [31] | |
| Breast | Serum | Inverse | HR | [40] | |
| Serum | Inverse | HR | [41] | ||
| Lung | Serum | None | Qt | [42] | |
| Lung | Serum | Direct | HR | [43] | |
| Kidney | Serum | Inverse | OR | [44] | |
| Mouth | Intake | Direct | Qt | [25] | |
| Phosphorus | Brain | Intake | Direct | Qt | [24] |
| Prostate | Intake | None | RR | [45] | |
| Intake | Direct | Cr | [45] | ||
| Intake | Direct | OR | [46] | ||
| Intake | None | OR | [47] | ||
| Colon | Intake | Inverse # | RR | [48] | |
| Bladder | Intake | None | OR | [49] | |
| Mouth | Intake | Direct | Qt | [25] | |
| Calcium | Prostate | Tissue | Direct | Qt | [33] |
| Brain | Intake | Direct | Qt | [24] | |
| Ovary | Serum | Direct | Qt | [50] | |
| Breast | Serum | Inverse | HR | [51] | |
| Mouth | Serum | Direct | Qt | [26] | |
| Iron | Any | Serum | Direct/Inverse | HR | [52] |
| Intake | Direct | HR | [53] | ||
| Stomach | Tissue | Direct | Qt | [54] | |
| Brain | Intake | Direct | Qt | [24] | |
| Mouth | Intake | Direct | Qt | [25] | |
| Serum | Direct | Qt | [26] | ||
| Breast | Serum | None | HR | [55] | |
| Thyroid | Urine | Direct | OR | [56] | |
| Iodine | Breast | Urine | Direct | Qt | [57] |
| Rectum | Tissue | Direct | Cr | [58] |
| Mineral | Cancer Entity | Sample | Case Group (Cancer) Mean Mineral Content Number of Patients |
Control Group (Healthy) Mean Mineral Content Number of Patients |
Reference |
|---|---|---|---|---|---|
| Zinc | Breast | Tissue † | 3.5–19.5 ppm (n = 26) | 0.8–11.4 ppm (n = 26) | [22] |
| Glioblastoma | Tissue | 0.0403 µg/cm2 (n = 11) | 0.0285 µg/cm2 (n = 11) | [24] | |
| Tissue † | 0.20 g/kg (n = 6) | 0.27 g/kg (n = 6) | [23] | ||
| Colon | Serum | 96.4 µg/dL (n = 966) | 97.1 µg/dL (n = 966) | [29] | |
| Copper | Glioblastoma | Tissue | 0.0090 µg/cm2 (n = 11) | 0.0079 µg/cm2 (n = 11) | [24] |
| Tissue † | 0.48 g/kg (n = 6) | 1.26 g/kg (n = 6) | [23] | ||
| Serum | 27.5 µmol/L (n = 52) ‡ | 19.7 µmol/L (n = 52) ‡ | [30] | ||
| Colon | Serum | 138.6 µg/dL (n = 966) | 135.8 µg/dL (n = 966) | [29] | |
| Pancreas | Serum | 1432 µg/L (n = 100) | 1098 µg/L (n = 100) | [31] | |
| Selenium | Any | Serum | 58.8 µg/L * | 84.8 µg/L (n = 966) | [36][39] |
| Esophageal | Tissue † | 0.73 µg/g (n = 30) ‡ | 0.59 µg/g (n = 30) ‡ | [32] | |
| Prostate | Tissue | 191 µg/kg (n = 49) | 168 µg/kg (n = 49) | [33] | |
| Serum | 0.13 µg/g (n = 467) | 0.14 µg/g (n = 936) | [34] | ||
| Breast | Serum | 90.5 ng/mL (n = 100) | 91.3 ng/mL (n = 1186) | [40] | |
| Liver | Serum | 67.47 µg/L (n = 187) | 108.38 µg/L (n = 120) | [37] | |
| Colon | Serum | 84.0 µg/L (n = 966) | 85.6 µg/L (n = 966) | [39] | |
| Pancreas | Serum | 60.0 µg/L (n = 100) | 76.0 µg/L (n = 100) | [31] | |
| Lung | Serum | 166.00 ng/g (n = 48) | 144.74 ng/g (n = 39) | [42] | |
| Renal | Serum | 161.7 µg/L (n = 401) | 288.8 µg/L (n = 774) | [44] | |
| Phosphorus | Glioblastoma | Tissue | 1.71 µg/cm2 (n = 11) | 3.01 µg/cm2 (n = 11) | [24] |
| Iron | Glioblastoma | Tissue | 0.037 µg/cm2 (n = 11) | 0.118 µg/cm2 (n = 11) | [24] |
| Oral | Serum | 194.6 µg/dL * | 128.6 µg/dL * | [26] | |
| Calcium | Prostate | Tissue | 657 mg/kg (n = 50) | 1431 mg/kg (n = 49) | [33] |
| Oral | Serum | 14.7 mEq/L * | 9.4 mEq/L * | [26] | |
| Ovary | Serum | 9.34 mg/dL (n = 170) | 9.31 mg/dL (n = 344) | [50] |
| Mineral | Cancer Entity | Case Group (Cancer) Mean Mineral Intake Number of Patients |
Control Group (Healthy) Mean Mineral Intake Number of Patients |
Reference |
|---|---|---|---|---|
| Zinc | Oral | 12,851 µg/day (n = 27) | 11,788 µg/day (n = 86) | [25] |
| Bladder | 14.5 mg/day (n = 198) | 14.7 mg/day (n = 377) | [49] | |
| Copper | Bladder | 2.5 mg/day (n = 198) | 2.8 mg/day (n = 377) | [49] |
| Selenium | Oral | 142.9 µg/day (n = 27) | 166.7 µg/day (n = 86) | [25] |
| Phosphorus | Oral | 1761 mg/day (n = 27) | 1431 mg/day (n = 86) | [25] |
| Bladder | 1898.3 mg/day (n = 198) | 1940.4 mg/day (n = 377) | [49] | |
| Iron | Oral | 22.4 mg/day (n = 27) | 18.9 mg/day (n = 86) | [25] |
| Bladder | 21.3 mg/day (n = 198) | 23.1 mg/day (n = 377) | [49] | |
| Calcium | Bladder | 1127.2 mg/day (n = 198) | 1194.5 mg/day (n = 377) | [49] |
3. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/cancers14051256
References
- Swinburn, B.; Sacks, G.; Ravussin, E. Increased Food Energy Supply Is More than Sufficient to Explain the US Epidemic of Obesity. Am. J. Clin. Nutr. 2009, 90, 1453–1456.
- Swinburn, B.A.; Sacks, G.; Hall, K.D.; McPherson, K.; Finegood, D.T.; Moodie, M.L.; Gortmaker, S.L. The Global Obesity Pandemic: Shaped by Global Drivers and Local Environments. Lancet Lond. Engl. 2011, 378, 804–814.
- Thomas, D. A Study on the Mineral Depletion of the Foods Available to Us as a Nation over the Period 1940 to 1991. Nutr. Health 2003, 17, 85–115.
- Thomas, D. The Mineral Depletion of Foods Available to Us as a Nation (1940–2002)—A Review of the 6th Edition of McCance and Widdowson. Nutr. Health 2007, 19, 21–55.
- Mayer, A.-M.B.; Trenchard, L.; Rayns, F. Historical Changes in the Mineral Content of Fruit and Vegetables in the UK from 1940 to 2019: A Concern for Human Nutrition and Agriculture. Int. J. Food Sci. Nutr. 2021, 1–12.
- Muthayya, S.; Rah, J.H.; Sugimoto, J.D.; Roos, F.F.; Kraemer, K.; Black, R.E. The Global Hidden Hunger Indices and Maps: An Advocacy Tool for Action. PLoS ONE 2013, 8, e67860.
- Biesalski, H.K.; Tinz, J. Multivitamin/Mineral Supplements: Rationale and Safety—A Systematic Review. Nutrition 2017, 33, 76–82.
- Shenkin, A. Micronutrients in Health and Disease. Postgrad. Med. J. 2006, 82, 559–567.
- Calder, P.C. Feeding the Immune System. Proc. Nutr. Soc. 2013, 72, 299–309.
- Shakoor, H.; Feehan, J.; Al Dhaheri, A.S.; Ali, H.I.; Platat, C.; Ismail, L.C.; Apostolopoulos, V.; Stojanovska, L. Immune-Boosting Role of Vitamins D, C, E, Zinc, Selenium and Omega-3 Fatty Acids: Could They Help against COVID-19? Maturitas 2021, 143, 1–9.
- Ribatti, D. The Concept of Immune Surveillance against Tumors. The First Theories. Oncotarget 2017, 8, 7175–7180.
- European Food Safety Authority (EFSA). Summary of Tolerable Upper Intake Levels—Version 4. Overview on Tolerable Upper Intake Levels as Derived by the Scientific Committee on Food (SCF) and the EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). 2018; p. 2. Available online: https://www.efsa.europa.eu/sites/default/files/assets/UL_Summary_tables.pdf (accessed on 21 February 2022).
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Iron. EFSA J. 2014, 13, 4254.
- European Food Safety Authority (EFSA). Summary of Dietary Reference Values—Version 4. Overview on Dietary Reference Values for the EU Population as Derived by the EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). 2017; p. 5. Available online: https://www.efsa.europa.eu/sites/default/files/assets/DRV_Summary_tables_jan_17.pdf (accessed on 21 February 2022).
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Zinc. EFSA J. 2014, 12, 3844.
- Yokokawa, H.; Fukuda, H.; Saita, M.; Miyagami, T.; Takahashi, Y.; Hisaoka, T.; Naito, T. Serum zinc concentrations and characteristics of zinc deficiency/marginal deficiency among Japanese subjects. J. Gen. Fam. Med. 2020, 21, 248–255.
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Selenium. EFSA J. 2014, 12, 3846.
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Phosphorus. EFSA J. 2014, 13, 4185.
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Calcium. EFSA J. 2015, 13, 4101.
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Copper. EFSA J. 2015, 13, 4253.
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Iodine. EFSA J. 2014, 12, 3660.
- Rusch, P.; Hirner, A.V.; Schmitz, O.; Kimmig, R.; Hoffmann, O.; Diel, M. Zinc Distribution within Breast Cancer Tissue of Different Intrinsic Subtypes. Arch. Gynecol. Obstet. 2021, 303, 195–205.
- Dehnhardt, M.; Zoriy, M.V.; Khan, Z.; Reifenberger, G.; Ekström, T.J.; Sabine Becker, J.; Zilles, K.; Bauer, A. Element Distribution Is Altered in a Zone Surrounding Human Glioblastoma Multiforme. J. Trace Elem. Med. Biol. Organ. Soc. Miner. Trace Elem. GMS 2008, 22, 17–23.
- Wandzilak, A.; Czyzycki, M.; Radwanska, E.; Adamek, D.; Geraki, K.; Lankosz, M. X-ray Fluorescence Study of the Concentration of Selected Trace and Minor Elements in Human Brain Tumours. Spectrochim. Acta Part. B At. Spectrosc. 2015, 114, 52–57.
- Secchi, D.G.; Aballay, L.R.; Galíndez, M.F.; Piccini, D.; Lanfranchi, H.; Brunotto, M. Red Meat, Micronutrients and Oral Squamous Cell Carcinoma of Argetine Adult Patients. Nutr. Hosp. 2015, 32, 1214–1221.
- Elango, S.; Samuel, S.; Khashim, Z.; Subbiah, U. Selenium Influences Trace Elements Homeostasis, Cancer Biomarkers in Squamous Cell Carcinoma Patients Administered with Cancerocidal Radiotherapy. Asian Pac. J. Cancer Prev. 2018, 19, 1785–1792.
- Fang, A.-P.; Chen, P.-Y.; Wang, X.-Y.; Liu, Z.-Y.; Zhang, D.-M.; Luo, Y.; Liao, G.-C.; Long, J.-A.; Zhong, R.-H.; Zhou, Z.-G.; et al. Serum Copper and Zinc Levels at Diagnosis and Hepatocellular Carcinoma Survival in the Guangdong Liver Cancer Cohort. Int. J. Cancer 2019, 144, 2823–2832.
- Tamai, Y.; Iwasa, M.; Eguchi, A.; Shigefuku, R.; Sugimoto, K.; Hasegawa, H.; Takei, Y. Serum Copper, Zinc and Metallothionein Serve as Potential Biomarkers for Hepatocellular Carcinoma. PLoS ONE 2020, 15, e0237370.
- Stepien, M.; Jenab, M.; Freisling, H.; Becker, N.-P.; Czuban, M.; Tjønneland, A.; Olsen, A.; Overvad, K.; Boutron-Ruault, M.-C.; Mancini, F.R.; et al. Pre-Diagnostic Copper and Zinc Biomarkers and Colorectal Cancer Risk in the European Prospective Investigation into Cancer and Nutrition Cohort. Carcinogenesis 2017, 38, 699–707.
- Turecký, L.; Kalina, P.; Uhlíková, E.; Námerová, S.; Krizko, J. Serum Ceruloplasmin and Copper Levels in Patients with Primary Brain Tumors. Klin. Wochenschr. 1984, 62, 187–189.
- Lener, M.R.; Scott, R.J.; Wiechowska-Kozłowska, A.; Serrano-Fernández, P.; Baszuk, P.; Jaworska-Bieniek, K.; Sukiennicki, G.; Marciniak, W.; Muszyńska, M.; Kładny, J.; et al. Serum Concentrations of Selenium and Copper in Patients Diagnosed with Pancreatic Cancer. Cancer Res. Treat. 2016, 48, 1056–1064.
- Xie, B.; Lin, J.; Sui, K.; Huang, Z.; Chen, Z.; Hang, W. Differential Diagnosis of Multielements in Cancerous and Non-Cancerous Esophageal Tissues. Talanta 2019, 196, 585–591.
- Çelen, İ.; Müezzinoğlu, T.; Ataman, O.Y.; Bakırdere, S.; Korkmaz, M.; Neşe, N.; Şenol, F.; Lekili, M. Selenium, Nickel, and Calcium Levels in Cancerous and Non-Cancerous Prostate Tissue Samples and Their Relation with Some Parameters. Environ. Sci. Pollut. Res. Int. 2015, 22, 13070–13076.
- Gill, J.K.; Franke, A.A.; Steven Morris, J.; Cooney, R.V.; Wilkens, L.R.; Le Marchand, L.; Goodman, M.T.; Henderson, B.E.; Kolonel, L.N. Association of Selenium, Tocopherols, Carotenoids, Retinol, and 15-Isoprostane F(2t) in Serum or Urine with Prostate Cancer Risk: The Multiethnic Cohort. Cancer Causes Control 2009, 20, 1161–1171.
- Coates, R.J.; Weiss, N.S.; Daling, J.R.; Morris, J.S.; Labbe, R.F. Serum Levels of Selenium and Retinol and the Subsequent Risk of Cancer. Am. J. Epidemiol. 1988, 128, 515–523.
- Brodin, O.; Hackler, J.; Misra, S.; Wendt, S.; Sun, Q.; Laaf, E.; Stoppe, C.; Björnstedt, M.; Schomburg, L. Selenoprotein P as Biomarker of Selenium Status in Clinical Trials with Therapeutic Dosages of Selenite. Nutrients 2020, 12, 1067.
- Kim, I.-W.; Bae, S.-M.; Kim, Y.-W.; Liu, H.-B.; Bae, S.H.; Choi, J.Y.; Yoon, S.K.; Chaturvedi, P.K.; Battogtokh, G.; Ahn, W.S. Serum Selenium Levels in Korean Hepatoma Patients. Biol. Trace Elem. Res. 2012, 148, 25–31.
- Gül-Klein, S.; Haxhiraj, D.; Seelig, J.; Kästner, A.; Hackler, J.; Sun, Q.; Heller, R.A.; Lachmann, N.; Pratschke, J.; Schmelzle, M.; et al. Serum Selenium Status as a Diagnostic Marker for the Prognosis of Liver Transplantation. Nutrients 2021, 13, 619.
- Hughes, D.J.; Fedirko, V.; Jenab, M.; Schomburg, L.; Méplan, C.; Freisling, H.; Bueno-de-Mesquita, H.B.; Hybsier, S.; Becker, N.-P.; Czuban, M.; et al. Selenium Status Is Associated with Colorectal Cancer Risk in the European Prospective Investigation of Cancer and Nutrition Cohort. Int. J. Cancer 2015, 136, 1149–1161.
- Sandsveden, M.; Nilsson, E.; Borgquist, S.; Rosendahl, A.H.; Manjer, J. Prediagnostic Serum Selenium Levels in Relation to Breast Cancer Survival and Tumor Characteristics. Int. J. Cancer 2020, 147, 2424–2436.
- Lubinski, J.; Marciniak, W.; Muszynska, M.; Huzarski, T.; Gronwald, J.; Cybulski, C.; Jakubowska, A.; Debniak, T.; Falco, M.; Kladny, J.; et al. Serum Selenium Levels Predict Survival after Breast Cancer. Breast Cancer Res. Treat. 2018, 167, 591–598.
- Callejón-Leblic, B.; Rodríguez-Moro, G.; Arias-Borrego, A.; Pereira-Vega, A.; Gómez-Ariza, J.L.; García-Barrera, T. Absolute Quantification of Selenoproteins and Selenometabolites in Lung Cancer Human Serum by Column Switching Coupled to Triple Quadrupole Inductively Coupled Plasma Mass Spectrometry. J. Chromatogr. A 2020, 1619, 460919.
- Pietrzak, S.; Wójcik, J.; Scott, R.J.; Kashyap, A.; Grodzki, T.; Baszuk, P.; Bielewicz, M.; Marciniak, W.; Wójcik, N.; Dębniak, T.; et al. Influence of the Selenium Level on Overall Survival in Lung Cancer. J. Trace Elem. Med. Biol. Organ. Soc. Miner. Trace Elem. GMS 2019, 56, 46–51.
- Hsueh, Y.-M.; Lin, Y.-C.; Huang, Y.-L.; Shiue, H.-S.; Pu, Y.-S.; Huang, C.-Y.; Chung, C.-J. Effect of Plasma Selenium, Red Blood Cell Cadmium, Total Urinary Arsenic Levels, and EGFR on Renal Cell Carcinoma. Sci. Total Environ. 2021, 750, 141547.
- Zhu, G.; Chen, C.; Hu, B.; Yuan, D.; Chen, W.; Wang, W.; Su, J.; Liu, Z.; Jiao, K.; Chen, X.; et al. Dietary Phosphorus Intake and Serum Prostate-Specific Antigen in Non-Prostate Cancer American Adults: A Secondary Analysis of the National Health and Nutrition Examination Survey (NHANES), 2003–2010. Asia Pac. J. Clin. Nutr. 2020, 29, 322–333.
- Wilson, K.M.; Shui, I.M.; Mucci, L.A.; Giovannucci, E. Calcium and Phosphorus Intake and Prostate Cancer Risk: A 24-y Follow-up Study. Am. J. Clin. Nutr. 2015, 101, 173–183.
- Tavani, A.; Bertuccio, P.; Bosetti, C.; Talamini, R.; Negri, E.; Franceschi, S.; Montella, M.; La Vecchia, C. Dietary Intake of Calcium, Vitamin D, Phosphorus and the Risk of Prostate Cancer. Eur. Urol. 2005, 48, 27–33.
- Kesse, E.; Boutron-Ruault, M.-C.; Norat, T.; Riboli, E.; Clavel-Chapelon, F. Dietary Calcium, Phosphorus, Vitamin D, Dairy Products and the Risk of Colorectal Adenoma and Cancer among French Women of the E3N-EPIC Prospective Study. Int. J. Cancer 2005, 117, 137–144.
- Brinkman, M.T.; Buntinx, F.; Kellen, E.; Dagnelie, P.C.; Van Dongen, M.C.J.M.; Muls, E.; Zeegers, M.P. Dietary Intake of Micronutrients and the Risk of Developing Bladder Cancer: Results from the Belgian Case-Control Study on Bladder Cancer Risk. Cancer Causes Control 2011, 22, 469–478.
- Kelly, M.G.; Winkler, S.S.; Lentz, S.S.; Berliner, S.H.; Swain, M.F.; Skinner, H.G.; Schwartz, G.G. Serum Calcium and Serum Albumin Are Biomarkers That Can Discriminate Malignant from Benign Pelvic Masses. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2015, 24, 1593–1598.
- Wulaningsih, W.; Sagoo, H.K.; Hamza, M.; Melvin, J.; Holmberg, L.; Garmo, H.; Malmström, H.; Lambe, M.; Hammar, N.; Walldius, G.; et al. Serum Calcium and the Risk of Breast Cancer: Findings from the Swedish AMORIS Study and a Meta-Analysis of Prospective Studies. Int. J. Mol. Sci. 2016, 17, 1487.
- Wen, C.P.; Lee, J.H.; Tai, Y.-P.; Wen, C.; Wu, S.B.; Tsai, M.K.; Hsieh, D.P.H.; Chiang, H.-C.; Hsiung, C.A.; Hsu, C.Y.; et al. High Serum Iron Is Associated with Increased Cancer Risk. Cancer Res. 2014, 74, 6589–6597.
- Zacharski, L.R.; Chow, B.K.; Howes, P.S.; Shamayeva, G.; Baron, J.A.; Dalman, R.L.; Malenka, D.J.; Ozaki, C.K.; Lavori, P.W. Decreased Cancer Risk after Iron Reduction in Patients with Peripheral Arterial Disease: Results from a Randomized Trial. J. Natl. Cancer Inst. 2008, 100, 996–1002.
- Sawayama, H.; Iwatsuki, M.; Kuroda, D.; Toihata, T.; Uchihara, T.; Koga, Y.; Yagi, T.; Kiyozumi, Y.; Eto, T.; Hiyoshi, Y.; et al. Total Iron-Binding Capacity Is a Novel Prognostic Marker after Curative Gastrectomy for Gastric Cancer. Int. J. Clin. Oncol. 2018, 23, 671–680.
- Von Holle, A.; O’Brien, K.M.; Sandler, D.P.; Janicek, R.; Weinberg, C.R. Association Between Serum Iron Biomarkers and Breast Cancer. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2021, 30, 422–425.
- Hu, M.-J.; He, J.-L.; Tong, X.-R.; Yang, W.-J.; Zhao, H.-H.; Li, G.-A.; Huang, F. Associations between Essential Microelements Exposure and the Aggressive Clinicopathologic Characteristics of Papillary Thyroid Cancer. Biometals 2021, 34, 909–921.
- Malya, F.U.; Kadioglu, H.; Hasbahceci, M.; Dolay, K.; Guzel, M.; Ersoy, Y.E. The Correlation between Breast Cancer and Urinary Iodine Excretion Levels. J. Int. Med. Res. 2018, 46, 687–692.
- Fan, S.; Li, X.; Zheng, L.; Hu, D.; Ren, X.; Ye, Z. Correlations between the Iodine Concentrations from Dual Energy Computed Tomography and Molecular Markers Ki-67 and HIF-1α in Rectal Cancer: A Preliminary Study. Eur. J. Radiol. 2017, 96, 109–114.
- Rohatgi, A. WebPlotDigitizer: Version 4.5. 2021. Available online: https://automeris.io/WebPlotDigitizer/ (accessed on 21 February 2022).
- Erem, S.; Razzaque, M.S. Dietary Phosphate Toxicity: An Emerging Global Health Concern. Histochem. Cell Biol. 2018, 150, 711–719.
- Tulchinsky, T.H. Micronutrient Deficiency Conditions: Global Health Issues. Public Health Rev. 2010, 32, 243–255.
- Dwyer, J.T.; Woteki, C.; Bailey, R.; Britten, P.; Carriquiry, A.; Gaine, P.C.; Miller, D.; Moshfegh, A.; Murphy, M.M.; Smith Edge, M. Fortification: New Findings and Implications. Nutr. Rev. 2014, 72, 127–141.
- Best, C.; Neufingerl, N.; Del Rosso, J.M.; Transler, C.; van den Briel, T.; Osendarp, S. Can Multi-Micronutrient Food Fortification Improve the Micronutrient Status, Growth, Health, and Cognition of Schoolchildren? A Systematic Review. Nutr. Rev. 2011, 69, 186–204.
- Rowe, L.A. Addressing the Fortification Quality Gap: A Proposed Way Forward. Nutrients 2020, 12, 3899.
- Dewi, N.U.; Mahmudiono, T. Effectiveness of Food Fortification in Improving Nutritional Status of Mothers and Children in Indonesia. Int. J. Environ. Res. Public Health 2021, 18, 2133.
- Das, J.K.; Salam, R.A.; Kumar, R.; Bhutta, Z.A. Micronutrient Fortification of Food and Its Impact on Woman and Child Health: A Systematic Review. Syst. Rev. 2013, 2, 67.
