Spent lithium batteries can cause pollution to the soil and seriously threaten the safety and property of people. They contain valuable metals, such as cobalt and lithium, which are nonrenewable resources, and their recycling and treatment have important economic, strategic, and environmental benefits. The hydrometallurgy process uses reagents such as hydrochloric acid (HCl), nitric acid (HNO3), sulfuric acid (H2SO4), phosphoric acid (H3PO4), organic acids, and hydrogen peroxide (H2O2) to extract and separate the cathode metals, usually operating below 100 °C, and can recover lithium in addition to the other transition metals.
- spent cathode material
- lithium-ion battery
- recycling
1. Introduction
The hydrometallurgy process uses reagents such as hydrochloric acid (HCl), nitric acid (HNO3), sulfuric acid (H2SO4), phosphoric acid (H3PO4), organic acids, and hydrogen peroxide (H2O2) to extract and separate the cathode metals, usually operating below 100 °C, and can recover lithium in addition to the other transition metals.
This paper reviewed various hydrometallurgy methods developed in the last decade for the recovery of cathode materials for lithium-ion batteries from various battery chemicals, such as LCO, LMO, NCM, and LFP, for the recovery of cobalt, nickel, manganese, and lithium.
As shown in Figure 1, hydrometallurgy works to crush and dissolve spent batteries, and then uses suitable chemical reagents to selectively separate the metal elements in the leaching solution, yielding high-grade cobalt metal or lithium carbonate. Hydrometallurgy is more suitable for recycling spent LIBs with single chemical composition, and its equipment investment cost is low, suitable for the recycling of small- and medium-scale spent LIBs. Therefore, this method is widely used at present.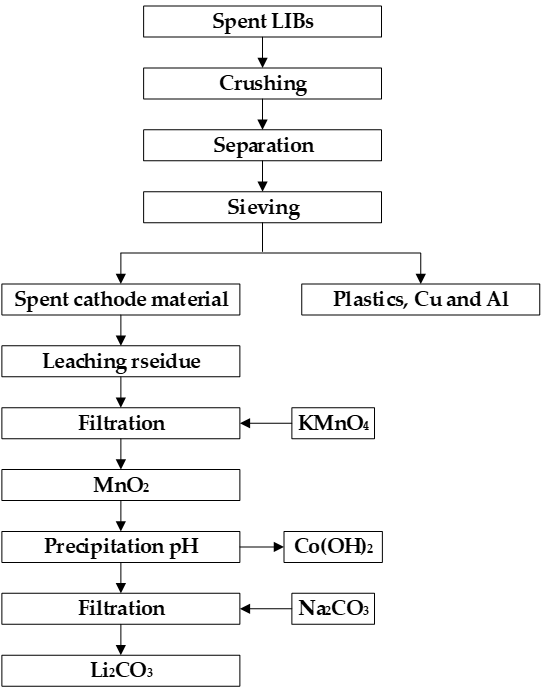
Figure 1. Flow chart of the hydrometallurgical process for recovering battery cathode material.
The valuable metals, mainly lithium, cobalt, and manganese, are extracted from the spent LIB cathode material by the acid leaching process, as concluded in Table 1.
Once the valuable metal has been leached, the metal can be recovered through a series of processes including precipitation and solvent extraction. After the leaching process, the recovered material can be reformulated to regenerate the lithium cathode material.
Table 1. Several major acid leach recovery processes.
|
Leaching Treatment |
Auxiliary Means |
Leach Temp |
Leach Efficiency (%) |
Reference |
|||
|
Inorganic Leaching Agent |
Reducing Agent |
|
|
Li |
Co |
Mn |
|
|
4 mol/L HCl |
|
|
80 °C |
99 |
99 |
|
[1] |
|
4 mol/L HCl |
|
|
80 °C |
99 |
99 |
99 |
[2] |
|
2 mol/L H2SO4 |
|
|
|
76 |
76 |
|
[3] |
|
2 mol/L H2SO4 |
H2O2 |
|
80 °C |
99 |
99 |
|
[3] |
|
2 mol/L H2SO4 |
H2O2 |
|
|
|
99.9 |
|
[4] |
|
4 mol/L H2SO4 |
H2O2 |
|
70 °C |
99 |
99 |
100 |
[5] |
|
2 mol/L H2SO4 |
H2O2 |
ultrasound |
30 °C |
98.6 |
94.6 |
|
[6] |
|
1 mol/L HNO3 |
|
|
75 °C |
75 |
40 |
|
[7] |
|
1 mol/L HNO3 |
|
|
75 °C |
75 |
40 |
|
[8] |
|
1 mol/L HNO3 |
H2O2 |
|
75 °C |
95 |
95 |
|
[9] |
|
1.5mol/L H3PO4 |
C6H12O6 |
|
80 °C |
98 |
100 |
|
[10] |
|
1.5mol/L malic acid |
grape seed |
|
80 °C |
99 |
92 |
|
[11] |
|
10 mol/L HCOOH |
H2O2 |
|
80 °C |
99.5 |
|
|
[12] |
|
0.5mol/L ascorbic acid |
|
microwave |
125 °C |
100 |
100 |
100 |
[13] |
|
57.8%(v/v) lemon juice |
H2O2 |
ultrasound |
40 °C |
100 |
96 |
96 |
[14] |
|
citric acid |
|
microwave |
|
|
|
94 |
[15] |
|
H2C2O4-H3PO4 |
|
|
|
100 |
98.2 |
100 |
[16] |
|
H2SO4-FePO4·2H2O |
|
|
80 °C |
96 |
96 |
96 |
[17] |
|
0.5 mol/L HCl- 0.5mol/L ascorbic acid |
|
|
90 °C |
97.72 |
97.25 |
|
[18] |
|
0.5 mol/LCH3COOH- 0.2mol/L ascorbic acid |
bagasse pith |
ultrasound |
50 °C |
98 |
98 |
98 |
[19] |
2. Acid Leaching
Most of the active cathode materials in LIBs can be dissolved in acid. Thus, the pretreated electrode material can be leached with an acid solution to achieve separation of the active material from the collector fluid, and then combined with the principle of a neutralization reaction to precipitate and purify the target metal, thereby achieving recovery of high-purity components. Acid solutions utilized by the acid leaching method are conventional inorganic acids, including hydrochloric acid, sulfuric acid, and nitric acid.
The leaching of spent LIBs using inorganic strong acids generates large amounts of spent liquids, as well as some harmful gases such as chlorine gas (Cl2) and sulfur trioxide (SO3), which can pollute the environment. These spent liquids are difficult in terms of being treated harmlessly. Researchers have tried to treat spent LIBs using organic acids, such as malic acid, citric acid, oxalic acid, malic acid, and ascorbic acid.
The selective recovery of the metal from the leachate is carried out in multiple steps. First, the Mn in the leachate reacts selectively with the KMnO4 reagent and nears comple-tion, and the Mn is recovered as MnO2 and manganese hydroxide. Second, nickel in the leachate is selectively extracted by dimethylglyoxime near completion. Third, the pH is adjusted by using a sodium hydroxide solution to selectively precipitate cobalt hydroxide. The addition of a saturated Na2CO3 solution precipitated Li2CO3. The purity of lithium, manganese, cobalt, and nickel is 96.97 wt%, 98.23 wt%, 96.94 wt%, and 97.43 wt%, respec-tively.
Almost all leaching processes require large amounts of acid and the reducer/oxidizer to achieve the desired leaching results, with acid concentrations ranging from 1.0 M to 3.0 M and high consumption of the reducer/oxidizer (e.g., 2–6 vol% hydrogen peroxide). Furthermore, large amounts of acid or the reductant/oxidizer are practically ineffective for accurate recovery of the target metal, and any unreacted acid or reductant/oxidizer ends up in the effluent and causes secondary contamination. It can be observed that different additives are used to reduce or oxidize the corresponding spent cathode materials (e.g., reductants for LCO and oxidizers for LFP), resulting in a single batch of different types of cathode materials that cannot be recovered synergistically. Therefore, the balance between simplifying the recycling process and saving chemical/energy consumption should be fully considered in order to pursue the efficient and green recycling of different metals from spent LIBs. Unlike conventional leaching processes that require reducing or oxidizing agents, the different redox properties of LCO and LFP are fully utilized to avoid the use of additional reducing or oxidizing agents. Moreover, due to the intrinsic motivation of the redox reactions of LCO and LFP and the transformation of transition metals, especially Fe, the amount of acid is presumed to be significantly reduced. The dissolved metals in the leachate can then be recovered efficiently and selectively as different products depending on the differences in solubility.
This entry is adapted from the peer-reviewed paper 10.3390/en15051611
References
- Zhang, P.W.; Yokoyama, T.; Itabashi, O.; Suzuki, T.M.; Inoue, K., Hydrometallurgical process for recovery of metal values from spent lithium-ion secondary batteries. Hydrometallurgy 1998, 47, (2-3), 259-271.
- Wang, R.; Lin, Y.; Wu, S., A novel recovery process of metal values from the cathode active materials of the lithium-ion secondary batteries. Hydrometallurgy 2009, 99, (3-4), 194-201.
- Sun, L.; Qiu, K., Vacuum pyrolysis and hydrometallurgical process for the recovery of valuable metals from spent lithium-ion batteries. J Hazard Mater 2011, 194, 378-384.
- Cheng, J.; Lu, T.; Huang, S.; Li, G.; Wang, J.; Kong, F.; Cheng, Q.; Zhang, Y., Recovery of cobalt from spent lithium-ion batteries as the dopant of TiO2 photocatalysts for boosting ciprofloxacin degradation. J Clean Prod 2021, 316, 128279.
- Min, X.; Guo, M.; Liu, L.; Li, L.; Gu, J.; Liang, J.; Chen, C.; Li, K.; Jia, J.; Sun, T., Synthesis of MnO2 derived from spent lithium-ion batteries via advanced oxidation and its application in VOCs oxidation. J Hazard Mater 2021, 406, 124743.
- Jiang, F.; Chen, Y.; Ju, S.; Zhu, Q.; Zhang, L.; Peng, J.; Wang, X.; Miller, J.D., Ultrasound-assisted leaching of cobalt and lithium from spent lithium-ion batteries. Ultrason Sonochem 2018, 48, 88-95.
- Castillo, S.; Ansart, F.; Laberty-Robert, C.; Portal, J., Advances in the recovering of spent lithium battery compounds. J Power Sources 2002, 112, (1), 247-254.
- Lee, C.K.; Rhee, K.I., Preparation of LiCoO2 from spent lithium-ion batteries. J Power Sources 2002, 109, (1), 17-21.
- Lee, C.K.; Rhee, K.I., Reductive leaching of cathodic active materials from lithium ion battery wastes. Hydrometallurgy 2003, 68, (1-3), 5-10.
- Meng, Q.; Zhang, Y.; Dong, P., Use of glucose as reductant to recover Co from spent lithium ions batteries. Waste Manage 2017, 64, 214-218.
- Zhang, Y.; Meng, Q.; Dong, P.; Duan, J.; Lin, Y., Use of grape seed as reductant for leaching of cobalt from spent lithium-ion batteries. J Ind Eng Chem 2018, 66, 86-93.
- Zeba, G.T.C.; Paulino, J.F.; Afonso, J.C., Recovery of metals from electroactive components of spent Li-ion batteries after leaching with formic acid. Braz J Chem Eng 2021.
- Lie, J.; Liu, J., Closed-vessel microwave leaching of valuable metals from spent lithium-ion batteries (LIBs) using dual-function leaching agent: Ascorbic acid. Sep Purif Technol 2021, 266.
- Esmaeili, M.; Rastegar, S.O.; Beigzadeh, R.; Gu, T., Ultrasound-assisted leaching of spent lithium ion batteries by natural organic acids and H2O2. Chemosphere 2020, 254.
- Pindar, S.; Dhawan, N., Microwave processing of spent coin cells for recycling of metallic values. J Clean Prod 2021, 280, (2).
- Tao, H.; Yang, Y.; Xu, S.; Liu, Q.; Huang, G.; Xu, Z., A lattice defect-inspired leaching strategy toward simultaneous recovery and separation of value metals from spent cathode materials. Waste Manage 2021, 135, 40-46.
- Liu, Y.; Lv, W.; Zheng, X.; Ruan, D.; Yang, Y.; Cao, H.; Sun, Z., Near-to-Stoichiometric Acidic Recovery of Spent Lithium-Ion Batteries through Induced Crystallization. Acs Sustain Chem Eng 2021, 9, (8), 3183-3194.
- Xing, L.; Bao, J.; Zhou, S.; Qiu, Y.; Sun, H.; Gu, S.; Yu, J., Ultra-fast leaching of critical metals from spent lithium-ion batteries cathode materials achieved by the synergy-coordination mechanism. Chem Eng J 2021, 420, (1).
- Yan, S.; Sun, C.; Zhou, T.; Gao, R.; Xie, H., Ultrasonic-assisted leaching of valuable metals from spent lithium-ion batteries using organic additives. Sep Purif Technol 2021, 257, 117930.
