3.1. Epidemiology, Etiology, and Risk Factors
According to data from 2021, every year, about 932,000 new HNC cases are registered and there are about 467,000 HNC deaths [
4]. Neoplasms originating in this anatomical area are some of the most common cancers worldwide, as HNC cases represent about 6% of all cases of cancer [
23,
24,
25,
26,
27]. The incidence of HNC varies depending on geographic region, population, gender (more common in men), and exposure to diverse risk factors. The major risk factors include tobacco, alcohol consumption, and human papillomavirus (HPV) infection, these being recognized as the main causes of upper aerodigestive cancers in industrialized regions [
11,
13,
24,
26,
28,
29,
30]. However, HNC results from several factors, including genetic predisposition, environmental exposure, and behavioral/lifestyle factors. The use of tobacco and alcohol is responsible for about 72% of all HNCs, 4% being due to the use of alcohol alone, 33% to tobacco alone, and 35% to the combined use of these. Thus, the cooccurrence of smoking and alcohol consumption increases the chance of developing HNC, though smoking is considered to be a more major risk factor than alcohol for this type of cancer [
29,
31]. That said, alcohol is a trigger for the tobacco promoter effect in neoplasm formation [
21]. After smoking cessation, there is a reduction in relative risk; however, an individual who was a heavy smoker has triple the risk of a non-smoker, even after 10 years of cessation [
21].
Some occupational and environmental contexts have been related to increased incidence of HNC, such as agricultural activities and working a as a cook, waiter, firefighter, butcher or meat preparer, knitter, or roofer. These associations exist because these work environments and occupations are more conducive to smoking and/or alcohol consumption [
30]. HPV infection, particularly subtype 16, and to a slighter extent, subtype 18, is believed to be a risk factor for oropharyngeal cancer, based on results in a young non-smoking population [
26,
29]. Patients with oropharyngeal cancer initiated by the virus typically showed better therapeutic results, and consequently a higher overall survival rate; thus, it is possible to note that age may function as a protective factor, being the reason for the increased survival of this group. Additionally, the augmented expression of p16 protein in HPV-related tumors has significantly better disease-specific survival when compared with non-virus-related tumors that do not exhibit increased p16 protein expression [
29]. Infection with this virus promotes an uncontrolled cell cycle which results in genetic instability which, over time, promotes the transformation of premalignant lesions into invasive squamous cell carcinomas. In the case of oropharyngeal squamous cell carcinoma, the stage of development of the virus is an independent prognostic factor for overall survival and progression-free survival [
32,
33].
Epidemiological studies show that, although the previously-mentioned risk factors are the main ones for most HNCs, nasopharyngeal cancers usually present a set of common etiological factors that include, in addition to those described above, Epstein–Barr virus (EBV) infection and processed food [
24,
34]. EBV is a DNA lymphotropic herpesvirus that is responsible for the presence of infectious mononucleosis and is highly prevalent in healthy individuals, affecting more than 90% of individuals worldwide [
35]. This virus is not found in tumor cells exclusively; however, it is not present in normal cells of the nasopharyngeal epithelium, which implies a direct relationship between EBV activation and the pathogenesis of the tumor [
34]. Regarding the co-presence of the above-mentioned viral infections and HNC, a study performed by Al-Thawadi et al. showed that HPV and EBV oncoviruses are co-present in squamous cell carcinomas, especially when they occur in the oral cavity, which may promote their initiation and/or progression; however, the mechanisms of this relationship need to be better elucidated [
36].
3.2. Pathophysiology
Understanding the origin and pathophysiology of the HCN is fundamental to predicting and managing the course of the disease, and its impact on the patient’s quality of life. This process facilitates the choice of the most appropriate treatment or combination (surgery, radiation therapy, and chemotherapy) while also minimizing possible sequels, such as significant acute and chronic damage to the oral cavity, which is not limited to the hard tissue (teeth and alveolar bone) and the oral mucous membrane, but also affects the soft tissues of the head and neck [
37]. Generally, all these malignancies are epithelial because they develop on the upper layers of the epidermis (mucosa) of the upper aerodigestive tract, squamous cell carcinoma being the most common histological type of head and neck tumor. These tumors can range from poorly to well differentiated, and in fact, about 90% of all HNC are squamous cell carcinomas and variants [
23,
28,
38].
In the presence of premature lesions, leucoplakia and erythroplakia with histologic features of hyperplasia or dysplasia are evident. Both cases may deform into invasive tumors, but erythroplakia presents a higher risk of transformation [
39]. As in other types of cancer, malignant cells also escape recognition and destruction by immune agents and inhibit or manipulate antitumor immune defenses. It is therefore common for patients with these types of cancer to have low concentrations of CD3+, CD4+, and CD8+ T cells, which may persist even several years after curative surgery. The main mechanisms of immune escape used by tumors to grow and target immune cells are immune destruction escape, tumor suppressor escape and cell regulation, reduction of T lymphocyte activity, immunosuppressive cells, and cytokines that control local and systemic effects [
28].
Since the affected areas in these types of cancers are adjacent to the respiratory and digestive systems, the same agents that promote the development of cancer cells in HNCs also affect other organs throughout the body, including the lungs. Thus, these tumors may not appear in isolation, but rather in association with other secondary tumors [
40]. As in other types of cancer, angiogenesis is also a determining factor in the development of neoplasm and progression of tumors and is regulated by several endogenous proangiogenic and antiangiogenic factors. Fundamental factors for the growth of cancer and metastasis are the vascular endothelial growth factor (VEGF) and its receptors. This receptor can be upregulated and has significant importance in the prognosis of several HNCs [
22,
28]. Another important marker that has high expression in HNC is the epidermal growth factor receptor (EGFR), which is expressed in more than 90% of tumors. EGFR is highly expressed in normal epithelial cells, so alterations in its pathways can promote a malignant transformation of HNC [
32]. Their expression levels correlate with worsened disease-free survival and overall survival [
22,
32].
HNC can also result from mutations in various genes and pathways, including both tumor-suppressor genes and oncogenes. Some biomarkers aid in the screening, diagnosis, and management of the disease. TP53 and CDKN2A/P16 are mutated tumor-suppressor genes frequently observed in HNC that may confer growth advantages to cells and encourage the development of carcinoma. FAT1 is one of the latest genes implicated in HNC that participates in cell cycle regulation and proliferation, and it is described as a tumor suppressor gene. NOTCH1 is the most recent cancer gene associated with HNC development. Functionally, the gene signaling has both oncogenic and tumor-suppressive roles depending on the cellular context; however, its exact role in pathogenesis needs to be better elucidated. The RAS gene family involves three oncogenes whose mutations in their cell cascades are included in approximately one-quarter of all human cancers. The PIK3CA pathway is another critical pathway in HNC carcinogenesis [
32]. Another marker that is sometimes overexpressed in HNC is programmed death-ligand 1 (PD-L1), a transmembrane protein that acts as a co-inhibitory factor of the immune response, reducing the patient’s immune response to tumor cells. Its presence is thus associated with a negative prognosis [
41,
42]. Advancements in the knowledge of these molecular structures (receptors) and genetic changes which are biomarkers for HNC are important, as they can be potential targets for therapy and help to define new diagnostic and therapeutic strategies, namely, those concerning personalized therapeutics.
3.4. Diagnosis and Treatment
For the diagnosis of a patient with upper aerodigestive tract complaints, it is essential to understand which areas of the head and neck may cause the symptoms. This procedure is fundamental because the extent of the tumor influences the prognosis and treatment [
22,
33]. An accurate and early diagnosis is one of the main strategies for successful management of HNC; however, most head and neck tumors are locally advanced at the time of diagnosis, even though they can be easily detected by simple physical examinations [
11,
22,
24]. An evaluation by a multidisciplinary team of the patients medical history, lifelong tobacco and alcohol consumption habits, and the past existence of other cancers and their treatments, including radiotherapy should be questioned [
21,
33].
The discovery and control of HNC is not always an easy task, since often the affected structures are not accessible to objective clinical examination, resulting in late presentation of the disease. Thus, if any symptom is suspected, an objective inspection of all the structures that may be involved should be performed, including examination of the oral cavity and oropharynx, palpation of the neck, and examination of suspect areas in the mouth. These patients should also undergo transnasal fiber optic endoscopy to examine the pharynx, larynx, and vocal cord structure [
21]. Diagnosis will allow evaluating the prognosis of the patient, since it helps to establish the TNM (tumor, node, metastasis) profile of the tumor. TNM staging is commonly used to assess the stages of tumors of the head and neck (although variations are depending on the site of the primary tumor) (
Table 1). The “T” classifies the extent of the primary tumor, the “N” refers to the infected regional lymph nodes (it is important to note that the lymphatic drainage of each head and neck subsite is different, so this must be assessed according to the location of the primary lesion), and the “M” refers to distant metastases. With this analysis, it is also possible to establish the stage of the disease, which can vary from 0 to IV, the latter being the stage with the worst prognosis [
11,
43].
Table 1. Tumor classification according to TNM profile [
39,
43]. T—extent of the primary tumor, N—infected regional lymph nodes, M—distant metastases.
| Stage |
T |
N |
M |
| 0 |
Defined shape |
No invasion |
No distant metastasis |
| I |
Defined shape, less than 2 cm
Does not invade the submucosa |
No invasion |
No distant metastasis |
| II |
Between 2 and 4 cm
Initial invasion of the submucosa |
No invasion |
No distant metastasis |
| III |
Cancer cells rapidly divide
Tumors with more than 4 cm |
Invasion |
No distant metastasis |
| IV |
Cancer cells enter the bloodstream |
Invasion |
Distant metastasis |
In recent years there has been great progress in defining the staging of head and neck tumors and therapeutic strategies. However, despite all the advances made in the various treatment modalities, the survival rate has remained almost unchanged in the last 25 years. Additionally, since head and neck anatomy are extremely complex, both being composed of several interconnected and interdependent structures, and since typically HNC occurs near structures that are important both at functional and cosmetic levels, early diagnosis is imperative [
43].
Despite recent advances in diagnosis, approximately 70% of patients with head and neck squamous cell carcinoma (HNSCC) present with advanced-stage disease, frequently involving regional lymph nodes at the time of diagnosis, leading to high associated mortality. The 5-year survival rate is about 60% [
26,
28]. Late diagnosis usually implies that the cancer has infiltrated surrounding tissues and spread to the regional nodes, this sometimes being the only clinical manifestation. Furthermore, the invasion of surrounding structures allows the entrance of cancer cells into the bloodstream, which may enable the appearance of distant metastasis and secondary sites; however, distant metastases are not usually present (only in about 10% of patients) [
11,
24,
28,
44]. One of the biggest problems associated with this disease is its rate of recurrence. Approximately 50% to 60% of patients with localized HNSCC have their disease progress within two years after diagnosis, which drastically decreases the survival rate (from 80% to 50–35%—depending on the degree of disease progression). Patients with recurrent or metastatic disease have an estimated survival of less than one year [
24,
26].
Late diagnosis requires aggressive treatments with high morbidity. Most of the time, the treatment will compromise the organs necessary to perform simple functions, such as eating, breathing, and speaking [
24,
44]. Given the vital importance of the structures involved in these tumors, the therapeutic strategies adopted should not only aim to improve the survival rate, but also preserve the functions of the organs, indicating the need for a multidisciplinary approach [
26,
28]. The standard treatment for HNC involves surgical resection and radiotherapy (in combination or as isolated treatments) in early stages, and chemotherapy is used in advanced stages of the disease [
26,
38]. Concurrent chemo-radiation allows preservation of organ function, and it is the main treatment for tumors arising in the oropharynx, nasopharynx, laryngopharynx, and larynx. For oral cavity cancers, the highest cure rates are achieved by using surgical techniques with adjuvant or post-operative radiotherapy (associated or not with chemotherapy) [
25]. Radiation therapy is also important in the control and palliation of symptoms in patients with advanced/incurable HNC, allowing tumor reduction, prevention of ulceration and bleeding, and pain control [
45]. However, due to the complex anatomy of this region, the conventional approach is always limited, as the treatments can result in severe functional impairment. Surgical resection is usually inadequate due to anatomical limitations, so despite various attempts to improve the existing treatments, they still have severe side effects. The traditional surgical approach is always the preferred treatment, as it removes all macroscopic tumors, yet there is always the concern of having a microscopic disease. With this approach, it is common that malignant cells persist in the tissue margins adjacent to the surgery, meaning that microscopic disease is normally present in the margins of the surgical area, which is often associated with local recurrence and a poor prognosis. Thus, in most cases, radiotherapy is used as well. In addition, surgery can cause severe side effects, resulting in the loss of basic functions and the need for tracheostomy and/or gastrostomy. If a tumor invades the carotid artery or pre-vertebral tissues, it cannot be removed [
21,
38]. Therefore, if possible, radiation therapy is ideal, due to the reduction in the associated morbidity [
38]. This type of treatment can be applied both to the primary tumor and to the lymphatic nodes, and can be referred to as organ-preserving therapy, with or without chemotherapy. However, although radiotherapy is a non-invasive treatment, it is not innocuous, as it can lead to both acute and chronic toxicity in normal tissues. For example, radiotherapy to the head and neck region may cause undesirable radiotherapy-induced changes in the surrounding tissues; and side effects such as oral mucositis, hyposalivation, loss of taste, dental caries, dysphagia, dermatitis (acute) osteoradionecrosis, vessels stenosis, hypothyroidism, hearing loss (late), and trismus, thereby negatively impacting the patient’s quality of life [
25,
38].
Regarding chemotherapy, this treatment modality is not usually employed as an isolated treatment for HNC, but it can still be used in some scenarios and for different purposes [
11,
38]:
-
Radiation enhancement/synchronous chemotherapy, used in conjunction with radiation therapy—reduces the risk of lymph node metastasis;
-
Neo-adjuvant/induction chemotherapy—to reduce the tumor’s size before the main treatment;
-
Adjuvant—acts on the small lesions that cannot be removed by surgery, reduces the recurrence rate, and improves the survival rate;
-
Palliation—for distant metastases.
Currently, the main chemotherapy drugs used in the treatment of HNC are antimetabolites, platinum compounds, taxanes like fluorouracil (5-FU), methotrexate (MTX), cisplatin, carboplatin, docetaxel, and paclitaxel; and various therapeutic protocols can be used, depending on the evolution and stage of the disease [
11,
46]. One of the standard combinations used to treat recurrent/metastatic HNC non-expressing PD-L1 is cisplatin/5-FU/cetuximab, which allows an increase in the speed of response to therapy, although toxicity may also be higher than the alternatives [
47,
48]. In the case of chemotherapy induction, the standard regime is cisplatin (100 mg/m
2) on days 1, 22, and 43 of concomitant radiotherapy [
48]. The combination of docetaxel (75 mg/m
2) and low doses of cisplatin (75 mg/m
2) and 5-fluorouracil (750 mg/m
2) each day, for five consecutive days, is also used. This strategy has been shown to reduce the progression of distant metastases, particularly in high-risk patients [
49]. In addition to these classes of compounds, EGFR inhibitors have also emerged as a new treatment strategy for HNC. Another method is using certain antibodies that can recognize receptors in cancer cell membranes, leading targeted cell death. Cetuximab (2006) was the first monoclonal antibody to be approved, and demonstrated considerably improved overall survival in patients with locally advanced and recurrent or metastatic HNC tumors. It also showed the role EGFR signaling pathways play in the treatment of HNC [
28,
32,
50]. Nivolumab (2016) was the second antibody approved by the Food and Drug Administration (FDA) for cases of metastatic or recurrent HNSCC. More recently, in 2019, came the approval of pembrolizumab as a first-line treatment for patients with unresectable metastatic or recurrent HNSCC [
50].
When used in combination with radiotherapy, drugs have more severe and longer lasting side effects than when used alone. This strategy is used for advanced tumors and is reported to be superior to surgery or radiotherapy alone by 6–8% in terms of 5 year survival [
21,
25,
51]. Despite the advances made recently, focused on advanced treatments to preserve organ function and improve quality of life, most of these treatments for HNC have low efficacy, detrimental side effects, and associated morbidities, such as systemic toxicity and cosmetic damage due to lack of selectivity of the therapeutic agents and the invasiveness of the surgical procedures [
26,
52]. Most of the chemotherapy drugs lack specificity to tumor cells. Thus, they have negative effects on healthy cells as well, resulting in severe side effects, and usually, the concentration of drug achievable at the target is limited, resulting in suboptimal treatment [
11,
52]. In this sense, the patients subjected to these therapies require significant support from a multidisciplinary team for psychological and physical rehabilitation, including a speech and language therapist, a dietician, a restorative dentist, and a hygienist. However, after treatment, some side effects can persist, despite intensive rehabilitation. A small number of patients never return to a normal oral diet [
21]. For that reason, research has been focusing on targeted cancer therapies to prevent these effects.
The progress in immune checkpoint inhibitors (ICIs) for HNC came to change the therapeutic landscape of the disease, and led to a remarkable benefit for some patients. These are a class of drugs that bind to proteins present in cell membranes that are produced by immune cells such as T cells and some cancer cells, having the ability to block them. These proteins function as checkpoints, allowing the differentiation of self from non-self antigens, and when blocked, can facilitate signaling and mobilization for cell death by circulating T-lymphocytes. The main checkpoint proteins found in cancer cells involved in this type of response in HNC are PD-L1 and CTLA-4 [
50]. This therapeutic strategy in combination with other conventional ones, can generate long-lasting immune responses and may lead to a significant improvement in therapeutic efficacy and survival in patients with advanced HNC. An example of this therapeutic approach is pembrolizumab, an anti-PD-L1 antibody that has been approved as a first-line treatment for patients with recurrent or metastatic HNC. It was beneficial in some cases; however, only 20% of patients with advanced HNC who received it showed effective responses [
53]. Additionally, the efficacy of these monoclonal antibody therapies is higher when the patients have PD-L1-expressing tumors, making this therapeutic quite specific [
48]. Additionally, the majority of patients present primary resistance to ICIs, have several immune-related adverse events, and do not benefit from the use of these agents, emphasizing the need for developing predictive biomarkers to better determine who will benefit from treatment with ICIs and to reduce severe systemic toxicity [
53,
54].
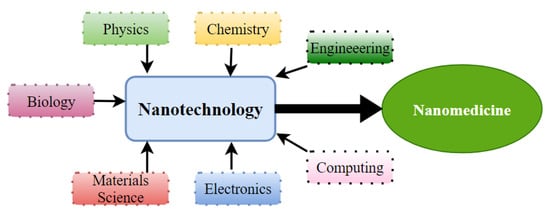
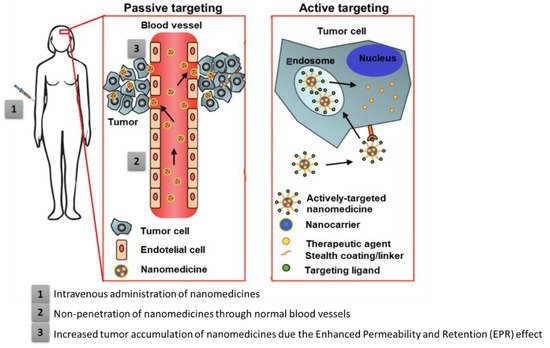
Moreover, like conventional cytotoxic therapy, immunotherapy is hampered by transport problems. Additionally, this difficulty of membrane permeation into solid tumor tissue by an ICI compromises the efficacy of such therapy. Therefore, it is urgently necessary to optimize the transport strategy of these therapeutic agents, namely, by using nanotechnology, as it can allow the transport of drugs selectively into tumor tissue, minimize toxicity in healthy tissues, and reduce immune-related side events.
Overall, given the drawbacks of these conventional treatments, the need has arisen to develop strategies and innovative technologies that improve the efficacy and safety of HNC therapies while reducing adverse effects and resistances.