Cancer is a group of disorders characterized by aberrant gene function and alterations in gene expression patterns. In 2020, it was anticipated that 19 million new cancer cases would be diagnosed globally, with around 10 million cancer deaths. Late diagnosis and interventions are the leading causes of cancer-related mortality. In addition, the absence of comprehensive cancer therapy adds to the burden. Many lyotropic non-lamellar liquid-crystalline-nanoparticle-mediated formulations have been developed in the last few decades, with promising results in drug delivery, therapeutics, and diagnostics. Cubosomes are nano-structured liquid-crystalline particles made of specific amphiphilic lipids in particular proportions. Their ability to encapsulate lipophilic, hydrophilic, and amphiphilic molecules within their structure makes them one of a kind. They are biocompatible, versatile drug carriers that can deliver medications through various routes of administration. Many preclinical studies on the use of cubosomes in cancer treatment and theranostic applications have been conducted. However, before cubosomes may be employed in clinical practice, significant technical advances must be accomplished.
1. Physiological Properites and Drug Delivery of Cubosome
The major advantage of cubosomes as nanoparticles is that they can accommodate hydrophilic, hydrophobic, and amphiphilic drug molecules. Cubosomes, according to the literature, have a number of other properties that make them attractive for use as drug-delivery vehicles. For example, they demonstrate biocompatibility; bio-adhesion; the protection of drug molecules against oxidation, hydrolysis, and deamidation processes; and the protection of protein molecules against denaturation, precipitation, aggregation, and surface adsorption. Additionally, they have been shown to be an effective delivery method over an extended period. These issues continue to be a barrier to achieving an optimal treatment response and patient compliance in the therapeutic region. Cubosomes are on the right track in this regard and have been suggested for use in a variety of chemical, peptide, and protein delivery systems.
Cubosomes’ physical properties make them ideal for oral drug delivery. Precipitation of oral medications is highly protected by cubosomes owing to the cubosomes’ lyotropic structure and consequent trapping of water-soluble compounds in the lipid bilayer absorption membrane. Another benefit of cubosomes in oral delivery is their potential to improve molecular absorption thanks to their bio-adhesive properties and surfactant production in the gastrointestinal system [
81,
82]. According to a study conducted by Mohsen et al., cubosomes have been demonstrated to increase the bioavailability of Coenzyme Q10, an antioxidant used in the treatment of liver disorders, by forming highly bioavailable and regulated drug formulations [
83]. Cubosome formulation, developed by Chung et al. previously, has successfully enhanced oral insulin absorption [
84].
The most significant problem with topical pharmaceutical delivery is enabling the medication to penetrate the skin. Several dosage forms have been produced to address this problem, and any penetration enhancers that have been developed have also been studied. While this is true, a critical difficulty still exists, which is the increase in the thermodynamic activity of active molecules without increasing their concentration in the skin, for the skin barrier to be more readily penetrated. Many studies have been successfully carried out to resolve these problems. Morsi and colleagues created cubosome formulations including silver sulfadiazine, monoolein, and the F127 stabilizer, which have been shown to be beneficial in treating deep second-degree burns [
85]. Furthermore, it has been demonstrated that colchicine manufactured as a cubosome transdermal preparation improves topical medication absorption, compared to when the drug is administered orally [
86].
Cubosomes have also shown considerable benefits in the delivery of drugs through intravenous and intranasal routes. Cubosomes may aid in the transfer of colloidal substances without obstructing capillaries. Additionally, they may minimize drug plasma–protein interactions, increasing drug molecules’ bioavailability and stability. Intranasal cubosomes can deliver drugs directly to the central nervous system (CNS) by crossing through the blood–brain barrier [
87]. Thus, cubosomes are a non-invasive medication delivery system for centrally acting drugs in various disorders. Cubosomes are beneficial in both routes of delivery. For example, Elsenosy and his team found that Duloxetine can be quickly delivered to the brain by in situ cubosome gel, which has better pharmacological effects [
88].
Cubosomes have been tested in many ways to exert their delivery capacity as nanoparticles with several disease models. However, very few studies have been conducted to evaluate the toxicity profiles of ingredients and stabilizers. Most studies of the sort have been tested with the help of in vitro analysis using MTT assays. Most of these studies evaluated the toxicity of phytantriol and monoolein-based cubosomes and stabilizers, such as Pluronics F108 and F127, and PEG conjugated lipids. The toxicity profiles for these materials were found to be very specific among the cell line chosen. For instance, F127 surfactants were found to be non-cytotoxic up to a concentration of 25 µg/mL in A549 and CHO cells, but were found to be highly toxic in HEK and L929 cells [
89,
90]. At the same time, F108 was found to be nontoxicnontoxic in Hela and HEK 293 cells up to 80 µg/mL concentrations [
91]. Hinton et al. compared the effects of F127 and the lipids monoolein and phytantriol on toxicity using an Alamar Blue assay. phytantriol-based cubosomes were found to be more toxic than monoolein-based ones. It was concluded that the cubic phase and its constituent lipid are the primary sources of toxicity, not the Pluronic [
92]. Murgia and co-workers found that while F127 itself is nontoxic, they speculate that monoolein promotes the internalization of F127 by decreasing its hydrophilicity and that, once internalized, its amphiphilic nature allows it to exert toxic activity towards the mitochondrial and other nuclear membranes [
93]. Studies carried out so far undoubtedly showed that the cubosome formulation’s toxicity may be due to the ingredients used in the formulation, or sometimes due to the drug or protein loaded in it. Hence formulation optimization and toxicity studies would need to be performed for individual cases.
2. Anticancer Activity of Cubosome
In cancer, the main challenges faced during the treatments are the targeted delivery of drugs to reduce the side effects and drug resistance by overcoming drug efflux transporters. Cubosomes are highly significantly taken into account in experiments in the cancer drug delivery area. They have achieved both targeted drug delivery and reduction in drug resistance. Moreover, as part of cancer drug treatment, cubosomes have also been used in immunotherapy. Studies have shown that cubosomes have improved the pharmacokinetics and safety profiles of the loaded drugs (Table 1).
Table 1. Applications of cubosomes for anticancer drug delivery.
| Sl No. |
Cancer/
Cells Type |
Chemicals/Drugs |
Polymer Used |
Stabilizer |
Findings |
Ref |
| 1 |
Colorectal/HCT-116 |
Cisplatin |
GMO |
Pluronic F127 |
Cisplatin-loaded nano-cubosomes decreased the cell viability of HCT 116 and augmentation of their cytotoxicity in the presence of metformin. |
[94] |
| 2 |
Colorectal/HCT-116 and Caco-2 |
Metformin |
GMO |
Pluronic F127 |
The cubosomes formulation significantly lowered the IC50 concentration at which viable cells were destroyed compared to metformin alone. |
[95] |
| 3 |
Colorectal/HT-29 |
Cornelian cherry |
GMO |
Poloxamer® 407 |
After 24 and 48 hours of incubation, Cornus mas extract cubosome improved IC50 value 1.33 and 1.47 times higher than free Cornus mas extract.
The cubosome formulation stopped G1 phase cell growth and produced apoptosis in the cancer cell line HT-29. |
[96] |
| 4 |
Colorectal/Caco-2 |
20(S)- protopanaxadiol |
GMO |
Poloxamer® 407 |
The PPD-cubosome showed higher bioavailability, and better release was which is likely owing to greater absorption by the cubic nanoparticles. |
[97] |
| 5 |
Hepatic/HepG2 |
5-Fluorouracil |
GMO |
Poloxamer® 407 |
5-FU-loaded cubosomes performed well in vitro cell culture. The cubosomes formulation also boosted bio distribution concentration of 5-FU in the liver compared to the 5-FU solution alone in the rat. |
[98] |
| 6 |
Hepatic/rat model |
Albendazole |
GMO |
Poloxamer® 407 |
The cubosome formulation of the drug resulted in a two-fold increase in bioavailability and greater tumor regression in a rat model of cancer. |
[99] |
| 7 |
Hepatic/SMMC-7721 |
Gambogenic acid |
GMO |
Poloxamer® 407 |
The prepared spherical or ellipsoidal monocellular cubosomes showed remarkable cytotoxicity in the SMMC-7721 cells. |
[100] |
| 8 |
Hepatic/HepG2 |
Resveratrol |
GMO |
Poloxamer® 407 |
The cubosome formulation had higher cytotoxicity against hepatic HepG2 cells in vitro, and superior cell internalization of drugs was observed. |
[35] |
| 9 |
Breast/MDA-MB-231 |
5- Fluorouracil |
Phytantriol |
Pluronic F127 |
In vitro cytotoxicity testing in the MDA-MB-231 cell line demonstrated that cubosomes containing 5-fluorouracil exhibit more cytotoxicity in the chosen cells than the medication alone. |
[101] |
| 10 |
Breast/MDA-MB-231/MCF-7 |
Thymoquinone |
GMO |
Poloxamer® 407 |
A dose and time-dependent increase in apoptotic cells was observed when treated with Thymoquinone-cubosome formulation against Thymoquinone alone. |
[102] |
| 11 |
Lung/A549 |
Bedaquiline |
GMO |
Poloxamer 188 |
The findings revealed that the cubosome formulation containing the medication exhibited considerable cytotoxicity in A549 cells, in addition to inducing apoptotic cell death, and had anti-invasive properties. |
[103] |
| 12 |
Lung/A549 |
Lumefantrine |
GMO |
Poloxamer |
In A549 cells, the cubosomes formulation demonstrated significantly greater anticancer and anti-angiogenesis action than the medication alone. |
[104] |
| 13 |
Cervical/Hela |
Doxorubicin |
GMO |
Pluronic F127 |
There was somewhat higher IC50 (15 MBq/mL) but statistically significant cytotoxicity at shorter time points, such as 24 h, with the cubosomes formulation. |
[105] |
| 14 |
Cervical/Hela |
Paclitaxel |
GMO |
PF108-B |
The biotinylated cubosome facilitated drug uptake at the cellular level. |
[106] |
| 15 |
Ovary/SKOV-3 and Caov 3 |
Icariin |
GMO |
Poloxamer® 407 |
The findings indicate that Icariin-cubosomes exhibit considerably increased cytotoxicity in both SKOV-3 and Caov 3 cells, but not in normal EA.hy926 endothelial cells. |
[107] |
| 16 |
Ovary/HEY |
Paclitaxel |
GMO |
Pluronic F127 |
The paclitaxel cubosomes demonstrated increased cytotoxicity in ovarian cells (HEY) and a 50% reduction in tumor burden in an animal xenograft model with more safety feature. |
[108] |
| 17 |
Skin/A431 cells |
Paclitaxel |
GMO |
Pluronic F127 |
Loaded paclitaxel accumulated preferentially at the tumor location. Additionally, when paclitaxel was loaded, the average tumor size was decreased to half of its original size when compared to the medication alone. |
[109] |
| 18 |
Skin/mice |
Resveratrol |
GMO |
Pluronic F127 |
The formulation improved skin permeability and deposition at the place of application in the mouse skin layer. |
[110] |
2.1. Cubosomes in Colorectal Cancer
Colorectal cancer is one of the most common and diagnosed solid malignancies globally. It appears in the colon or rectum mucosa as a malignant tumor [
111]. Based on the development, they have divided the process into five stages. Patients’ survival rates declined as the stages progressed, but the patient might be treated with surgery and chemotherapy if the diagnosis was made sooner. Despite this, medication resistance and adverse effects continue to be critical obstacles. Nanoparticle technology has been extensively employed for effective medication release, cancer cell targeting, and reducing chemotherapy side effects [
112].
The application of cubosomes nanoparticles has experimented with several times in colorectal cancer [
113,
114]. Saber and colleagues had explored reducing the toxicity of cisplatin, a major drug used in colorectal chemotherapy. They demonstrated significant anticancer efficacy in vitro against human colorectal cancer cells compared to unformulated cisplatin. In their study, nano cubosomes have prepared with GMO and Pluronic F127. According to the study, metformin’s cytotoxicity is increased when combined with nano-cubosome cisplatin. The IC
50 in colorectal HCT-116 cells after treatment with cisplatin was 15 µM, whereas the IC
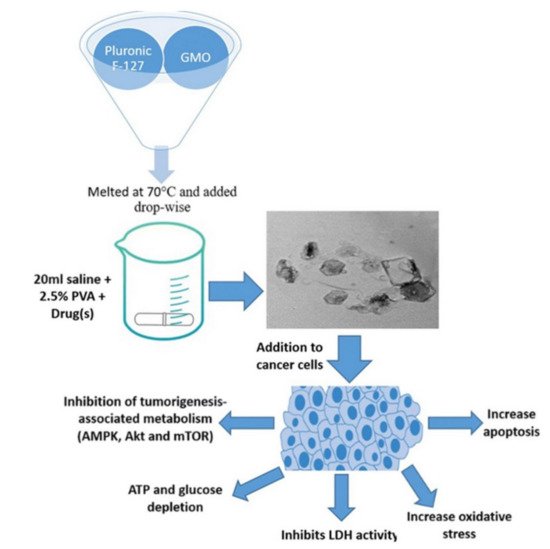
50 of cisplatin-loaded nano-cubosomes was 9.6 µM. Additionally, the determination of cisplatin concentrations at the intracellular level 48 h after treatment with the same concentration of (7 µM) cisplatin, cisplatin nano-cubosomes, and cisplatin-metformin nano-cubosomes revealed a significantly increased uptake of the drug due to nano-cubosomes drug incorporation. This was shown clearly in colon cells treated with nano-cubosomes, where a 1.6 fold increase in cisplatin concentration was found compared to untreated cells. Moreover, cubosome nanoparticles induced death in CRC cells by disrupting numerous metabolic pathways (e.g., mTOR inhibition, AMPK activation), lowering glucose levels, and decreasing energy levels (
Figure 4) [
94].
Figure 4. The emulsification process is used to manufacture cisplatin and cisplatin–metformin nanocubosomes. When CRC cells are treated with drug-loaded nanocubosomes, multiple metabolic pathways, including the AMPK/mTOR and Akt/mTOR pathways, are significantly inhibited. As a consequence of the depletion of ATP and glucose, there is a rise in oxidative stress and apoptosis. Another way the nano-cubosomes cause cytotoxicity is by inhibiting LDH activity, which leads to caspase-3 activation. Source: Reproduced from [
94].
Magdy et al. examined metformin alone in colorectal cancer. This anti-diabetic medication has already shown its ability to decrease the incidence of colorectal adenoma and thereby improve patient life. Magdy and colleagues developed a monoolein, and water-based metformin cubosome dispersion stabilized with Pluronic F127. The findings indicated that metformin-loaded cubosomes generated much more toxicity in vitro in HCT-116 and Caco-2 colorectal cancer cells than unloaded cubosomes or metformin alone. The cubosomes formulation significantly lowered the formulation’s IC
50 concentration at which viable cells were destroyed. In HCT-116 cells, the IC
50 decreased to 20 from 55 mM; however, in Caco-2 cells, it dropped to 28 from 50 mM. Their work demonstrates unambiguously the possibility of incorporating a modest amount of metformin into cubosomes to treat colorectal cancer patients [
95].
Aside from using synthetic medications in cubosome formulations, researchers have developed and described a range of natural products that have been integrated into cubosome preparations to study their effects on colon cancer. Anthocyaninsas is a naturally occurring bioactive phenolic constituent in Cornelian cherry (
Cornus mas L.) fruit. It has significant cytotoxic and antioxidant activity. Its oral bioavailability remains very low due to the destruction of chemicals and participation of gastro microbiota during their metabolism. Aside from that, it was discovered earlier that its increased absorption occurs via the small intestine. Radbeh and colleagues have created enteric-coated nano cubosomes of Cornelian cherry extracts to improve their anticancer activity in colon cancer cells and protect them from gastrointestinal damage. Cubosomes were created by combining glycerolmonooleate with the stabilizer poloxamer
® 407 in a proprietary formulation. The findings revealed that the cubosome prepared significantly protects the antioxidant activity of the extract. Its ability to induce apoptosis and cytotoxicity in HT-29 cells increased, with an inhibitory concentration (IC
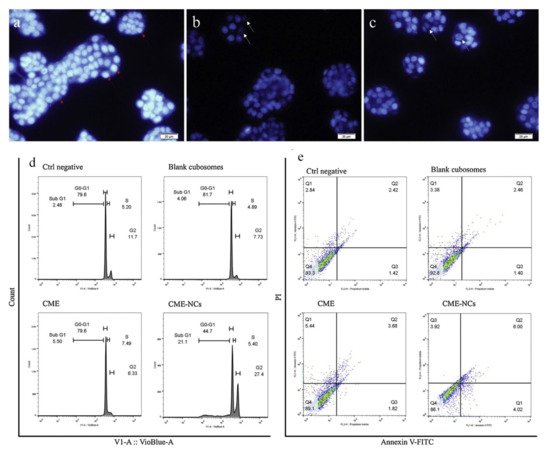
50 value) of 1.33 and 1.47 times greater than that of free Cornus mas extract after 24 and 48 h of incubation, respectively. Finally, they discovered that the increase in cell cycle arrest occurs during the G0-G1 phase, contributing to the reported loss in cell viability seen. G0-G1 phase could be a critical factor in the observed reduction in cell viability (
Figure 5) [
96].
Figure 5. Cornus mas extract-nano carrier induced apoptosis and cell cycle arrest: (
a) negative control, (
b) Cornus mas extract treatment; and (
c) Cornus mas extract treatment with nano carriers presented fluorescence pictures of treated and untreated DAPI stained HT-29 cells (red arrows indicate healthy cell nuclei and white arrows indicate fragmented cell nuclei samples); (
d) depicts cell cycle analysis, whereas (
e) depicts cell apoptosis as determined by Annexin V FITC/PI (propidium iodide) labeling. Source: Adapted with permission from [
96], Elsevier Masson SAS, 2020.
Ginsenosides are a family of natural steroid glycosides and triterpene saponins derived from Panax species. It has been demonstrated to have vast medicinal characteristics, especially in cancer. Ginsenosides is a prodrug that, following intestinal metabolism, transforms into an active product. 20(S)- protopanaxadiol (PPD) is Ginsenosides’ most active and effective anticancer metabolites. Despite being an effective substance for cancer, it is has a downside of weak water solubility and poor penetration of cancer cells. Jin and colleagues have sought to sidestep these pharmacological features by manufacturing cubosome preparations by fragmentation the glyceryl monoolein (GMO)/Poloxamer 407 bulk cubic gels. They investigated its numerous medicinal capabilities in the colon Caco-2 cells model. They have observed that PPD-cubosome formulation may raise the cell permeability apical to the basolateral of PPD at 53 %. This behavior may be attributed to the specific qualities of cubosomes produced, which severed as permeability enhancers and bioadhesive. Hence, the formulation has been proven to be a paradigm for colorectal cancer, notably by the oral route of delivery [
97].
2.2. Cubosomes in Liver Cancer
Liver cancer is one of the deadliest diseases globally, and in the USA, it is the 4th major reason for cancer-related mortality. It has a poor prognosis rate, and the primary causes for the disease include fatty liver, hepatitis, cirrhosis, obesity, etc. In the first stages, the surgical intervention appears to be beneficial to the patients; however, within the later stages, chemotherapy is adopted. There are limited alternatives to accessible chemotherapeutic medications like sorafenib. The risk of drug resistance to the regimen during the six months is a major concern. [
115]. To enhance liver cancer therapy, several novel nanomaterials have been employed presently. Their distinct physical properties might offer targeted drug delivery accuracy and reduced adverse effects. Several efforts have been made to include drugs in cubosomes to boost their therapeutic action.
5-Fluorouracil (5FU) is a potent anticancer drug that has been used to treat solid tumors, particularly liver cancer. However, the substantial adverse effects of 5FU, including hematologic and gastrointestinal complications, preclude its widespread usage in many circumstances [
98]. Thus, to reduce the therapeutic dose of 5FU and improve its physical properties, Nasr and colleagues synthesized cubosome dispersions and evaluated their effectiveness in vitro in human hepatoma HepG2 cells and in vivo in rats. They employed a cubic gel phase of monoolein, water, and Poloxamer 407 as a stabilizer. Their formulation demonstrated a quick release of around half of the entrapped 5FU during the first hour and a gradual release of the remainder. The biodistribution of 5FU in the rat liver was substantially greater in cubosome formulations than in 5FU alone. They could not detect a significant variation in the IC
50 in HepG2 cells during the in vitro cytotoxicity investigation. These findings indicated that the cubosome formulation of 5FU did not affect the drug’s cytotoxicity. The non-significant result suggested that the medication alone caused the observed cell toxicity, not by the cubosome particle [
116].
Albendazole is a potent inhibitor of numerous solid tumors. They are effective against hepatocellular cancer [
117]. Albendazole’s low bioavailability, however, remains a concern in malignancies. Albendazole inhibits cancer via interacting with microtubules and inhibiting tubulin formation. Saber and colleagues came up with a cubosome formulation to look into the possibility of better bioavailability and the likely mechanism of albendazole’s anticancer action. They created albendazole-loaded cubosome dispersions utilizing GMO and P407 stabilizers. The findings indicated that the cubosome formulation of the drug resulted in a two-fold increase in bioavailability and more significant tumor regression in a rat model of cancer. Their investigation revealed that albendazole might prevent liver cancer by altering the ERK1/2-HIF-1-p300/CREB pathways via the cubosome formulation [
99].
Natural products, such as extracts, isolated chemicals, and analogs, have been investigated as a possible medication or lead molecule in cancer treatment. Among these is gambogenic acid, a naturally occurring chemical derived from Gamboge, a herb used in traditional Chinese medicine. They demonstrated significant anticancer activity in preclinical tests through cell cycle arrest and cyclin D modulation. However, disadvantages such as poor solubility, short shelf life, irritation of blood vessels, and light sensitivity precluded it from clinical use. Luo and colleagues sought to synthesize gambogenic acid cubosomes using GMO and the stabilizer F127. They assessed its physiochemical characteristics and antitumor activity against SMMC-7721 human hepatocellular cancer cells. They successfully generated spherical or ellipsoidal monocellular cubosomes with exceptional cytotoxicity in SMMC-7721 cells. In vivo investigations have shown that cubosome formulations had a higher Cmax and AUC than the medication alone [
100]. Another natural substance, Resveratrol, is a stilbenoid, a natural phenol that has shown a strong affinity for hepatocellular carcinoma cells. As with gambogenic acid, resveratrol has been associated with limited water solubility, low bioavailability, and photosensitivity. Abdel-Bar and colleagues overcame these challenges by preparing resveratrol cubosomes using a GMO and a P407 stabilizer. The cytotoxicity experiment revealed that the cubosome formulation was more cytotoxic in vitro to hepatic HepG2 cells. Additionally, they found improved drug uptake by cells. [
35].
2.3. Cubosomes in Breast Cancer
Breast cancer is the leading cause of cancer in women globally. It is treatable in up to 80% of patients if found early and without metastases. It is difficult to treat cancer patients who have advanced to metastasis with current therapies. Breast cancer is classified as a heterogeneous illness at the molecular level due to epidermal growth factor receptor 2, hormone receptors, and many mutations [
118]. Breast cancer treatment plans are determined by their molecular subtypes and include surgical intervention and chemotherapy. There are inadequate medication penetration, low bioavailability, and stability as with other solid tumors. Numerous attempts have been made to solve the problems related to breast cancer therapy by incorporating nanoparticles [
119].
5- Fluorouracil is a commonly used medication in treating breast cancer, especially triple-negative breast cancer. As an antimetabolite of pyrimidine analog, it modulates several apoptotic pathways in breast cancer. It is quickly absorbed into systemic circulation through blood vessels, resulting in low drug concentration levels at the tumor site [
120]. This will result in a decrease in efficacy as well as increased toxicity. Astolfi and colleagues created a bulk phase cubosome dispersion for 5-fluorouracil. To prepare the cubosomes, they employed phytantriol and Pluronic F127 stabilizer. In vitro cytotoxicity testing in the MDA-MB-231 cell line demonstrated that cubosomes containing 5-fluorouracil have higher cytotoxicity in the chosen cells than the medication alone [
101].
Many naturally occurring chemicals have shown remarkable anti-breast cancer action. Thymoquinone is an active chemical derived from the plant
Nigella sativa that effectively treats breast cancer. Their clinical applicability is hampered by properties such as low bioavailability and the lack of a measurement procedure in blood and tissues. Mehanna and colleagues generated thymoquinone-loaded cubosomes using an emulsification homogenization process. They compared the effects on estrogen-positive MCF-7 and estrogen-negative MDA-MB-231 cells to normal breast cells (MCF-10A). Containing a mean particle size of 98 nm, a prepared cubosome with GMO and pluronic F127 stabilizer demonstrated excellent entrapment effectiveness. They discovered a dose-dependent and time-dependent increase in apoptotic cells when treated with cubosome formulation against thymoquinone alone. Furthermore, drug accumulation in cells was better in cubosomes containing drugs [
102].
2.4. Cubosomes in Lung Cancer
Lung cancer is becoming more common around the world. According to GLOBACAN, lung cancer became the most common disease and cancer-related mortality in 2019 when men and women were combined. With two major types: non–small-cell lung cancer (NSCLC) and small-cell lung cancer (SCLC), the most common cause of lung cancer is still smoking, although other variables such as asbestos and biomass burning air pollution are also linked to lung cancer. Despite several chemopreventive drugs being available for lung cancer, inconsistent outcomes make clinical recommendations challenging. Despite the availability of numerous modern medical techniques such as surgery, chemotherapy, and radiation therapy, treating lung cancer is getting more complicated as time passes. The main issue with chemotherapy in lung cancer is the lack of accuracy and the negative effects of therapeutic dosages. In this regard, nanotechnology plays a critical role in offering an appropriate delivery method [
121,
122].
Bedaquiline is a medicine that the FDA has licensed to treat TB, and it has shown great success in the inhibition of lung cancer. However, because of the drug’s limited water solubility, it has a significant difficulty in reaching the lungs. For this reason, Patil and colleagues created bedaquiline-loaded cubosomes that were specifically designed to target NSCLC. They made the inhalable cubosomes using GMO and the stabilizer Poloxamer 188. They could create a cubosome with a 51 percent encapsulation capacity and particle sizes of 150 nm. After nebulization, the cubosome showed excellent aerodynamic properties with a Mass median aerodynamic diameter 4.21 ± 0.53 µm. The cytotoxicity analysis revealed that the bedaquiline-loaded cubosomes exhibited considerable cytotoxicity in A549 cells through apoptosis. It is the first research to use cubosomes in inhalation treatment, and it is the most promising [
103].
Lumefantrine, or benflumetol, is a well-known antimalarial drug. Numerous investigations indicate that this medicine may be employed as an anticancer agent in various solid tumors. It has a few drug delivery issues, including limited solubility in water and low bioavailability. Sethuraman and colleagues produced cubosomes filled with Lumefantrine calcium phosphate nanoparticles to investigate site-specific delivery in lung cancer. They created the cubosome by monolinolein, pyridinylmethyl linoleate, and Poloxamer 188 stabilizers. The cubosomes had shown encapsulating capacity of 78 % and a particle size of 259 nm. In A549 cells, the cubosomes formulation demonstrated significantly greater anticancer and anti-angiogenesis action than the medication alone [
104].
2.5. Cubosomes in Cervical Cancer
Even though cervical cancer is the third most frequent disease in women, it is the most common cause of cancer in 40 low-income nations. It usually affects middle-aged women, and the major reason for this is the prevalence of the human papillomavirus (HPV). It is a condition that can be avoided, and getting the HPV vaccination at a young age may assist substantially with cervical cancer prevention. When a middle-aged woman is diagnosed with cervical cancer, treatment starts with surgical excision of early lesions, followed by chemotherapy and radiation. Cervical cancer therapy is currently costly, invasive, nonspecific, and unsatisfactory. As a result, Nanotechnology has been used to circumvent these problems, allowing for precision and target delivery with fewer adverse effects [
123].
Doxorubicin is a commonly used anticancer medicine that is particularly effective against cervical cancer. However, free radical development of doxorubicin–iron complexes in the bloodstream increases the drug’s toxicity. Co-administration of antioxidant treatment is being utilized to reduce its adverse effects. A combination of external irradiation and doxorubicin has been used to minimize the therapeutic dosage of doxorubicin and its negative effects. External radiotherapy may be used to sensitize ionizing radiation to radionuclides associated with chemotherapeutic drugs. Cytryniak and colleagues established a dual-modality drug delivery method in Hela cervical cells in vitro, employing cubosomes for internal irradiation, paired with doxorubicin. The system is composed of GMO, a Pluronic F127 stabilizer, and 177Lu, a radionuclide with a low-energy beta (β
−)-emitter. They discovered that cubosomes alone are non-toxic to Hela cells up to 54 µg/mL GMO. They detected a somewhat higher IC
50 (15 MBq/mL) but statistically significant cytotoxicity at shorter time points, such as 24 h, with the cubosomes formulation [
105].
Paclitaxel has been shown to be an effective chemotherapeutic agent for cervical cancer. The primary adjuvants utilized in the commercial formulation of paclitaxel for clinical use are polyethoxylated castor oil and dehydrated ethanol. Both of these contribute to the formulation’s homogeneity. However, this adjuvant combination is quite toxic. As a result, Aleandri and colleagues developed a cubosome formulation of paclitaxel to decrease toxicity and improve the site-of-action specificity. The cubosomes were generated utilizing GMO and the stabilizer PF108-B. The cubosome formulation was described, and its effectiveness was determined in Hela cells. The findings indicated that biotinylated cubosomes had more active functional biotin on the cell surface and exhibited more antitumor efficacy than paclitaxel alone. Additionally, the biotinylated cubosomes facilitated drug uptake at the cellular level. As a result, the formulation may be utilized in place of the traditional paclitaxel formulation to minimize adverse effects [
106].
2.6. Cubosomes in Ovarian Cancer
Ovarian cancer is the fourth leading cause of mortality from cancer in women. It usually occurs in postmenopausal women, and the mortality rate is significant owing to the late presentation of the disease in clinics. Cancers are often treated surgically with hormone therapy, immunotherapy, chemotherapy, and radiation. However, the high likelihood of treatment resistance, adverse side effects, and relapse complicate care. Thus, novel tactics in pharmaceutical development are critical for minimizing adverse effects and ensuring effective medication delivery to the target location [
124].
Icariin is an isolated phytoconstituent from the Chinese traditional medicinal herb
Herba epimedii. Previous investigation has investigated its impact on ovarian cancer and its ability to trigger apoptosis through the PI3K/AKT and Raf1/ERK1/2 signaling cell-death pathways [
125]. While it has a substantial anticancer effect in ovarian cancer, its low water solubility limits its bioavailability. Additionally, it has been shown that Icariin is metabolized into an inactive form by deglycosylation. As a result, Fahmy and colleagues improved Icariin-cubosomes and evaluated their efficiency in vitro in ovarian cells (SKOV-3 and Caov 3). Cubosomes were synthesized utilizing GMOs and a P407 stabilizer. The findings indicate that Icariin-cubosomes exhibit increased cytotoxicity in SKOV-3 and Caov 3 cells but not in normal EA.hy926 endothelial cells. The observed results might be a consequence of the chemicals’ increased solubility in the cubosome formulation [
107].
Paclitaxel has been used to treat aggressive ovarian cancer similarly to how it is used in cervical cancer. However, as previously discussed, the difficulties connected with paclitaxel, such as adjuvant toxicity and significant side effects, remain the same. Zhai and colleagues developed cubosome formulations containing paclitaxel to enhance the effectiveness, while minimizing its adverse effects. The formulation comprised GMO and Pluronic F127 stabilizer, and was functionalized with EGFR fragments to improve tumor site targeting. They noticed a substantial drug loading capacity in the cubosome formulation. Additionally, the paclitaxel cubosomes demonstrated increased cytotoxicity in ovarian cells (HEY) and a 50% reduction in tumor burden in an animal xenograft model. The cubosome paclitaxel formulation outperformed paclitaxel alone in animal survival throughout the study. This demonstrates the formulation’s exceptional safety qualities [
108].
2.7. Cubosomes in Skin Cancer
Skin cancer is a broad category that refers to various skin-related carcinomas, including basal cell carcinoma, cutaneous squamous cell carcinoma, and melanoma [
126]. It is the most prevalent kind of cancer among Caucasians, and the most apparent causes are exposure to UV radiation and an aging population. Chemotherapy is the most successful treatment option for a variety of cancers. Still, when it comes to skin cancer—particularly melanoma, the most common kind of skin cancer—chemotherapy becomes exceedingly unsuccessful and unsatisfying. The main rationale for this is medication resistance caused by the disease’s unique traits. In skin cancer, drug resistance develops either due to acquired resistance during cytostatic drugs or as a result of inherent resistance. Thus, the hurdles in treatment strategies include overcoming resistance and increasing the quantity of drugs reaching tumor regions [
127].
Cubosomes have been used to circumvent the difficulties associated with chemotherapy in skin cancer. Thus, Zhai and colleagues chose paclitaxel as the active ingredient in cubosome formulations, to evaluate human epidermal carcinoma A431 and an animal skin cancer xenograft model. The preparation was formulated using GMO and Pluronic F127, and its cytotoxicity was determined using A431 cells. In vitro cell assays revealed that the paclitaxel cubosome formulation was more tolerable than the medication alone. In the xenograft model, it was extremely evident that loaded paclitaxel accumulated preferentially at the tumor location. Additionally, when paclitaxel was loaded, the average tumor was decreased to half of its original size when compared to the medication alone [
109]. Similarly, another natural substance, resveratrol, has been evaluated in cubosome formulations for the treatment of skin cancer. Resveratrol has previously shown anti-melanoma efficacy, although performance was determined to be suboptimal because of limited bioavailability. Kurangi and colleagues synthesized Resveratrol-loaded Cubosomal Gel from GMO and evaluated it in the skin layer of mice. The formulation improved skin permeability and deposition at the place of application in the mouse skin layer. The bioavailability investigation indicated that this compound has good potential for skin localization [
110].
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics14030600