Transient Receptor Potential (TRP) channels constitute a large superfamily of polymodal channel proteins with diverse roles in many physiological and sensory systems that function both as ionotropic and metabotropic receptors. From the early days of TRP channel discovery, membrane lipids were suggested to play a fundamental role in channel activation and regulation. A prominent example is the Drosophila TRP and TRP-like (TRPL) channels, which are predominantly expressed in the visual system of Drosophila. Light activation of the TRP and TRPL channels, the founding members of the TRP channel superfamily, requires activation of phospholipase Cβ (PLC), which hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) into Diacylglycerol (DAG) and Inositol 1, 4,5-trisphosphate (IP3).
- Drosophila TRP channel
- TRPL channel
- phospholipase Cβ
- Diacylglycerol (DAG)
- poly unsaturated fatty acids (PUFAs)
- cholesterol
- ergosterol
1. Introduction
2. The Involvement of the Inositol-Lipid Signaling in TRP Channel Activation
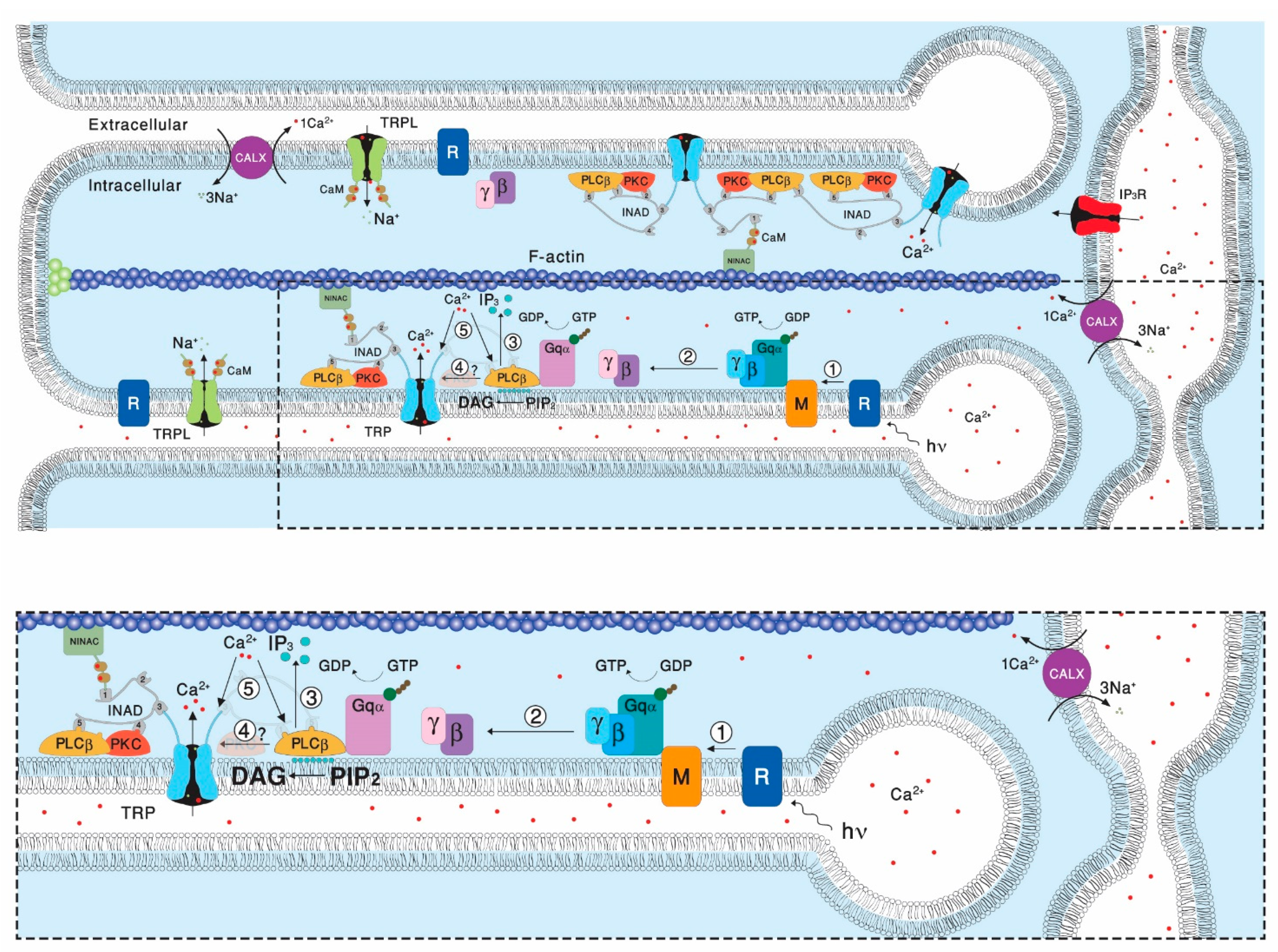
3. Lipid Composition of the Drosophila Head/Retina and the Effects of Its Modification
4. Evidence for Lipids Action as Second Messengers
5. PUFAs Activation of TRP and TRPL Channels in the Dark
6. Photomechanical Gating of the TRP/TRPL Channels
7. Lipid Rafts and Modulation of TRPL Channel Activity by Cholesterol
8. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/biom12030382
References
- Damann, N.; Voets, T.; Nilius, B. TRPs in our senses. Curr. Biol. 2008, 18, R880–R889.
- Hardie, R.C.; Raghu, P. Visual transduction in Drosophila. Nature 2001, 413, 186–193.
- Katz, B.; Minke, B. Drosophila photoreceptors and signaling mechanisms. Front. Cell. Neurosci. 2009, 3, 2.
- Kiselyov, K.; Patterson, R.L. The integrative function of TRPC channels. Front. Biosci. 2009, 14, 45–58.
- Minke, B.; Parnas, M. Insights on TRP channels from in vivo studies in Drosophila. Annu. Rev. Physiol. 2006, 68, 649–684.
- Barbera, N.; Ayee, M.A.A.; Akpa, B.S.; Levitan, I. Molecular Dynamics Simulations of Kir2.2 Interactions with an Ensemble of Cholesterol Molecules. Biophys. J. 2018, 115, 1264–1280.
- Yoshioka, T.; Inoue, H.; Hotta, Y. Defective phospholipid metabolism in the retinular cell membrane of norpA (no receptor potential) visual transduction mutants of Drosophila. Biochem. Biophys. Res. Commun. 1983, 111, 567–573.
- Yoshioka, T.; Inoue, H.; Hotta, Y. Absence of phosphatidylinositol phosphodiesterase in the head of a Drosophila visual mutant, norpA (no receptor potential A). J. Biochem. Tokyo 1985, 97, 1251–1254.
- Inoue, H.; Yoshioka, T.; Hotta, Y. Membrane-associated phospholipase C of Drosophila retina. J. Biochem. Tokyo 1988, 103, 91–94.
- Devary, O.; Heichal, O.; Blumenfeld, A.; Cassel, D.; Suss, E.; Barash, S.; Rubinstein, C.T.; Minke, B.; Selinger, Z. Coupling of photoexcited rhodopsin to inositol phospholipid hydrolysis in fly photoreceptors. Proc. Natl. Acad. Sci. USA 1987, 84, 6939–6943.
- Kohn, E.; Katz, B.; Yasin, B.; Peters, M.; Rhodes, E.; Zaguri, R.; Weiss, S.; Minke, B. Functional Cooperation between the IP3 Receptor and Phospholipase C Secures the High Sensitivity to Light of Drosophila Photoreceptors In Vivo. J. Neurosci. 2015, 35, 2530–2546.
- Pearn, M.T.; Randall, L.L.; Shortridge, R.D.; Burg, M.G.; Pak, W.L. Molecular, biochemical, and electrophysiological characterization of Drosohpila norpA mutannts. J. Biol. Chem. 1996, 271, 4937–4945.
- Cook, B.; Bar, Y.M.; Cohen-Ben, A.H.; Goldstein, R.E.; Paroush, Z.; Selinger, Z.; Minke, B. Phospholipase C and termination of G-protein-mediated signalling in vivo. Nat. Cell Biol. 2000, 2, 296–301.
- Waldo, G.L.; Ricks, T.K.; Hicks, S.N.; Cheever, M.L.; Kawano, T.; Tsuboi, K.; Wang, X.; Montell, C.; Kozasa, T.; Sondek, J.; et al. Kinetic Scaffolding Mediated by a Phospholipase C- and Gq Signaling Complex. Science 2010, 330, 974–980.
- Svobodova, B.; Groschner, K. Reprint of "Mechanisms of lipid regulation and lipid gating in TRPC channels". Cell Calcium 2016, 60, 133–141.
- Stark, W.S.; Lin, T.N.; Brackhahn, D.; Christianson, J.S.; Sun, G.Y. Fatty acids in the lipids of Drosophila heads: Effects of visual mutants, carotenoid deprivation and dietary fatty acids. Lipids 1993, 28, 345–350.
- Stark, W.S.; Lin, T.N.; Brackhahn, D.; Christianson, J.S.; Sun, G.Y. Phospholipids in Drosophila heads: Effects of visual mutants and phototransduction manipulations. Lipids 1993, 28, 23–28.
- Eroglu, C.; Brugger, B.; Wieland, F.; Sinning, I. Glutamate-binding affinity of Drosophila metabotropic glutamate receptor is modulated by association with lipid rafts. Proc. Natl. Acad. Sci. USA 2003, 100, 10219–10224.
- Rietveld, A.; Neutz, S.; Simons, K.; Eaton, S. Association of sterol- and glycosylphosphatidylinositol-linked proteins with Drosophila raft lipid microdomains. J. Biol. Chem. 1999, 274, 12049–12054.
- Julius, D. From peppers to peppermints: Natural products as probes of the pain pathway. Harvey Lect. 2005, 101, 89–115.
- Berridge, M.J. Inositol trisphosphate and calcium signalling. Nature 1993, 361, 315–325.
- Raghu, P.; Yadav, S.; Mallampati, N.B. Lipid signaling in Drosophila photoreceptors. Biochim. Biophys. Acta 2012, 1821, 1154–1165.
- Masai, I.; Okazaki, A.; Hosoya, T.; Hotta, Y. Drosophila retinal degeneration A gene encodes an eye-specific diacylglycerol kinase with cysteine-rich zinc-finger motifs and ankyrin repeats. Proc. Natl. Acad. Sci. USA 1993, 90, 11157–11161.
- Masai, I.; Suzuki, E.; Yoon, C.S.; Kohyama, A.; Hotta, Y. Immunolocalization of Drosophila eye-specific diacylgylcerol kinase, rdgA, which is essential for the maintenance of the photoreceptor. J. Neurobiol. 1997, 32, 695–706.
- Chyb, S.; Raghu, P.; Hardie, R.C. Polyunsaturated fatty acids activate the Drosophila light-sensitive channels TRP and TRPL. Nature 1999, 397, 255–259.
- Parnas, M.; Katz, B.; Lev, S.; Tzarfaty, V.; Dadon, D.; Gordon-Shaag, A.; Metzner, H.; Yaka, R.; Minke, B. Membrane lipid modulations remove divalent open channel block from TRP-like and NMDA channels. J. Neurosci. 2009, 29, 2371–2383.
- Lev, S.; Katz, B.; Tzarfaty, V.; Minke, B. Signal-dependent hydrolysis of phosphatidylinositol 4,5-bisphosphate without activation of phospholipase C: Implications on gating of Drosophila TRPL (transient receptor potential-like) channel. J. Biol. Chem. 2012, 287, 1436–1447.
- Leung, H.T.; Tseng-Crank, J.; Kim, E.; Mahapatra, C.; Shino, S.; Zhou, Y.; An, L.; Doerge, R.W.; Pak, W.L. DAG lipase activity is necessary for TRP channel regulation in Drosophila photoreceptors. Neuron 2008, 58, 884–896.
- Randall, A.S.; Liu, C.H.; Chu, B.; Zhang, Q.; Dongre, S.A.; Juusola, M.; Franze, K.; Wakelam, M.J.; Hardie, R.C. Speed and sensitivity of phototransduction in Drosophila depend on degree of saturation of membrane phospholipids. J. Neurosci. 2015, 35, 2731–2746.
- Delgado, R.; Muñoz, Y.; Peña-Cortés, H.; Giavalisco, P.; Bacigalupo, J. Diacylglycerol activates the light-dependent channel TRP in the photosensitive microvilli of Drosophila melanogaster photoreceptors. J. Neurosci. 2014, 34, 6679–6686.
- Lundbaek, J.A.; Collingwood, S.A.; Ingólfsson, H.I.; Kapoor, R.; Andersen, O.S. Lipid bilayer regulation of membrane protein function: Gramicidin channels as molecular force probes. J. R. Soc. Interface 2010, 7, 373–395.
- Hamill, O.P. Twenty odd years of stretch-sensitive channels. Pflugers Arch. 2006, 453, 333–351.
- Spassova, M.A.; Hewavitharana, T.; Xu, W.; Soboloff, J.; Gill, D.L. A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc. Natl. Acad. Sci. USA 2006, 103, 16586–16591.
- Simons, K.; Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000, 1, 31–39.
- Morenilla-Palao, C.; Pertusa, M.; Meseguer, V.; Cabedo, H.; Viana, F. Lipid raft segregation modulates TRPM8 channel activity. J. Biol. Chem. 2009, 284, 9215–9224.
- Wu, W.; Wang, Y.; Deng, X.L.; Sun, H.Y.; Li, G.R. Cholesterol down-regulates BK channels stably expressed in HEK 293 cells. PLoS ONE 2013, 8, e79952.
- Romanenko, V.G.; Fang, Y.; Byfield, F.; Travis, A.J.; Vandenberg, C.A.; Rothblat, G.H.; Levitan, I. Cholesterol sensitivity and lipid raft targeting of Kir2.1 channels. Biophys. J. 2004, 87, 3850–3861.
- Lichtenegger, M.; Tiapko, O.; Svobodova, B.; Stockner, T.; Glasnov, T.N.; Schreibmayer, W.; Platzer, D.; de la Cruz, G.G.; Krenn, S.; Schober, R.; et al. An optically controlled probe identifies lipid-gating fenestrations within the TRPC3 channel. Nat. Chem. Biol. 2018, 14, 396–404.
