The Enzyme-Linked Immunosorbent Assay is a versatile technique, which can be used for several applications. It has enormously contributed to the study of infectious diseases. This review highlights how this methodology supported the science conducted in COVID-19 pandemics, allowing scientists to better understand the immune response against SARS-CoV-2. ELISA can be modified to assess the functionality of antibodies, as avidity and neutralization, respectively by the standardization of avidity-ELISA and surrogate-neutralization methods. Cellular immunity can also be studied using this assay. Products secreted by cells, like proteins and cytokines, can be studied by ELISA or its derivative Enzyme-linked immunospot (ELISpot) assay. ELISA and ELISA-based methods aided the area of immunology against infectious diseases and is still relevant, for example, as a promising approach to study the differences between natural and vaccine-induced immune responses against SARS-CoV-2.
- ELISA
- immune response
- antibody
- cytokine
- SARS-CoV-2
1. Introduction
2. Detection of Immune Response to SARS-CoV-2
3. Enzyme-Linked Immunosorbent and Other Immunoassays
4. SARS-CoV-2 Antigens and Antibodies
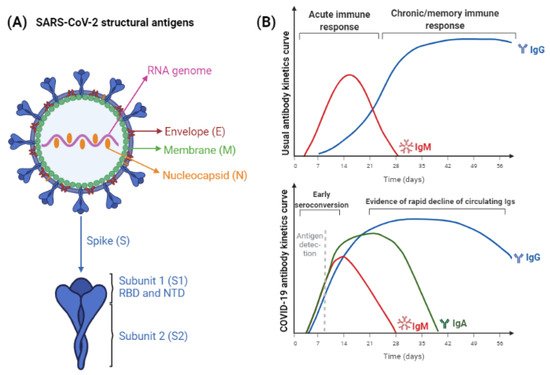
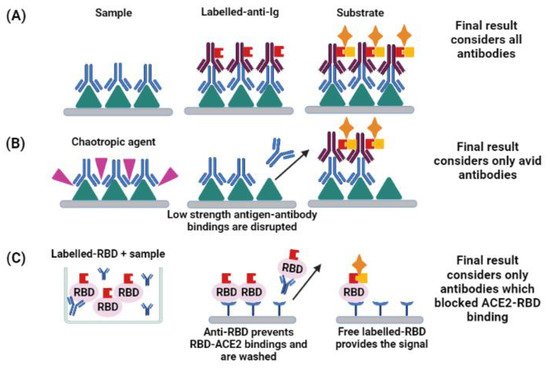
5. Functionality of Antibodies
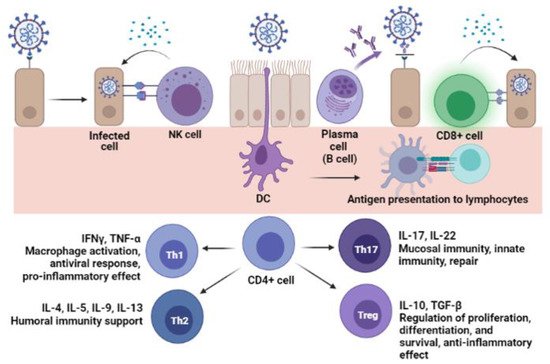
6. Cellular Immunity
| Cytokine | Secretor Cell | Immune Function | Effect on the Host | SARS-CoV-2 Association |
|---|---|---|---|---|
| CXCL-10 | Monocytes | Monocytes, macrophages, NK cells, DCs and LT chemotaxis | Inflammation | Severe disease [70] |
| Interferon-gamma (IFN-Υ) | NK cells and LT-CD4+ (Th1) | IL-4 inhibition, Th1 differentiation, increased MHC I and II expression | Inflammation and anti-viral immune response | Lung injury [71] |
| IL-1β | Macrophages | LB proliferation and differentiation, phagocytes stimulation | Inflammation | Decreased oxygen saturation, poor outcome [72,73] |
| IL-2 | Activated LT-CD4+, LB and monocytes | NK and T cell activation and proliferation, B cell activation along with IL-4 | Inflammation and antigen-specific stimulation | ICU-hospitalization [35,72] |
| IL-4 | LT-CD4+ (Th2) | LB differentiation and proliferation, increased expression of MHC-II | Antigen-specific humoral response | Mild disease [70] |
| IL-6 | Lymphocytes and monocytes | Increased acute inflammation-cytokines release, eosinophil chemotaxis | Immune modulation (pro or anti-inflammatory), antigen-specific response, and anti-viral response | Decreased oxygen saturation, poor outcome, increased risk of death [73,74] |
| IL-8 | Macrophages | Neutrophil and granulocytes chemotaxis, phagocytosis stimulation | Inflammation | Severe disease, increased risk of death [74,75] |
| IL-10 | LT-CD8+ | Inhibition of Th1 cytokines, decreased cytolytic response | Inflammation | Severe disease and ICU hospitalization [35,75] |
| IL-17 | LT-CD4+ (Th17) | Neutrophil activation | Inflammation, mucosal activation, tissue repair | Decreased oxygen-saturation and lung injury [71,73], mild disease [70] |
| Tumor-necrosis factor (TNF)-α | Macrophages | Phagocytes chemotaxis and phagocytosis stimulation | Inflammation | Severe disease, ICU hospitalization, and increased risk of death [35,74,75] |
7. Enzyme-Linked Techniques as Tools to Study Natural Infection and Vaccination
8. Conclusions
References
- Crowther, J.R. The ELISA Guidebook, 2nd ed.; Humana Press Inc.: Vienna, Austria, 2009; ISBN 978-1-60327-253-7. [Google Scholar]
- West, R.; Kobokovich, A.; Connell, N.; Gronvall, G.K. COVID-19 Antibody Tests: A Valuable Public Health Tool with Limited Relevance to Individuals. Trends Microbiol. 2021, 29, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Maggi, E.; Canonica, G.W.; Moretta, L. COVID-19: Unanswered questions on immune response and pathogenesis. J. Allergy Clin. Immunol. 2020, 146, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Assadiasl, S.; Fatahi, Y.; Zavvar, M.; Nicknam, M.H. COVID-19: Significance of antibodies. Hum. Antibodies 2020, 28, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Ong, D.S.Y.; Fragkou, P.C.; Schweitzer, V.A.; Chemaly, R.F.; Moschopoulos, C.D.; Skevaki, C.; European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Study Group for Respiratory Viruses (ESGREV). How to interpret and use COVID-19 serology and immunology tests. Clin. Microbiol. Infect. 2021, 27, 981–986. [Google Scholar] [CrossRef]
- Hamilton, R.G. The clinical immunology laboratory of the future. Clin. Chem. 1994, 40, 2186–2192. [Google Scholar] [CrossRef]
- Liu, G.; Rusling, J.F. COVID-19 Antibody Tests and Their Limitations. ACS Sensors 2021, 6, 593–612. [Google Scholar] [CrossRef]
- Amanat, F.; Stadlbauer, D.; Strohmeier, S.; Nguyen, T.H.O.; Chromikova, V.; McMahon, M.; Jiang, K.; Arunkumar, G.A.; Jurczyszak, D.; Polanco, J.; et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat. Med. 2020, 26, 1033–1036. [Google Scholar] [CrossRef]
- Moura, A.D.; Costa, H.H.M.; Correa, V.A.; Lima, A.K.S.; Lindoso, J.A.L.; De Gaspari, E.; Hong, M.A.; Cunha-Junior, J.P.; Prudencio, C.R. Assessment of avidity related to IgG subclasses in SARS-CoV-2 Brazilian infected patients. Sci. Rep. 2021, 11, 17642. [Google Scholar] [CrossRef]
- Allinson, J.L. Automated immunoassay equipment platforms for analytical support of pharmaceutical and biopharmaceutical development. Bioanalysis 2011, 3, 2803–2816. [Google Scholar] [CrossRef]
- Van Elslande, J.; Decru, B.; Jonckheere, S.; Van Wijngaerden, E.; Houben, E.; Vandecandelaere, P.; Indevuyst, C.; Depypere, M.; Desmet, S.; André, E.; et al. Antibody response against SARS-CoV-2 spike protein and nucleoprotein evaluated by four automated immunoassays and three ELISAs. Clin. Microbiol. Infect. 2020, 26, 1557.e1–1557.e7. [Google Scholar] [CrossRef]
- Byrum, J.R.; Waltari, E.; Janson, O.; Guo, S.-M.; Folkesson, J.; Chhun, B.B.; Vinden, J.; Ivanov, I.E.; Forst, M.L.; Li, H.; et al. multiSero: Open multiplex-ELISA platform for analyzing antibody responses to SARS-CoV-2 infection. MedRxiv 2021. [Google Scholar] [CrossRef]
- Krüttgen, A.; Cornelissen, C.G.; Dreher, M.; Hornef, M.; Imöl, M.; Kleines, M. Comparison of four new commercial serologic assays for determination of SARS-CoV-2 IgG. J. Clin. Virol. 2020, 128, 104394. [Google Scholar] [CrossRef] [PubMed]
- Olbrich, L.; Castelletti, N.; Schälte, Y.; Garí, M.; Pütz, P.; Bakuli, A.; Pritsch, M.; Kroidl, I.; Saathoff, E.; Guggenbuehl Noller, J.M.; et al. Head-to-head evaluation of seven different seroassays including direct viral neutralisation in a representative cohort for SARS-CoV-2. J. Gen. Virol. 2021, 102, 001653. [Google Scholar] [CrossRef]
- Bastos, L.M.; Tavaziva, G.; Abidi, S.K.; Campbell, J.R.; Haraoui, L.P.; Johnston, J.C.; Lan, Z.; Law, S.; MacLean, E.; Trajman, A.; et al. Diagnostic accuracy of serological tests for covid-19: Systematic review and meta-analysis. BMJ 2020, 370, m2516. [Google Scholar] [CrossRef] [PubMed]
- Saker, K.; Escuret, V.; Pitiot, V.; Massardier-Pilonchéry, M.; Paul, S.; Mokdad, B.; Langlois-Jaques, C.; Rabilloud, M.; Goncalves, D.; Fabien, N.; et al. Evaluation of commercial anti-SARS-CoV-2 antibody assays and comparison of standardized titers in vaccinated health care workers. J. Clin. Microbiol. 2021, 60, e01746-21. [Google Scholar] [CrossRef]
- Van Elslande, J.; Houben, E.; Depypere, M.; Brackenier, A.; Desmet, S.; André, E.; van Ranst, M.; Lagrou, K.; Vermeersch, P. Diagnostic performance of seven rapid IgG/IgM antibody tests and the Euroimmun IgA/IgG ELISA in COVID-19 patients. Clin. Microbiol. Infect. 2020, 26, 1082–1087. [Google Scholar] [CrossRef]
- Perez-Saez, J.; Zaballa, M.E.; Yerly, S.; Andrey, D.O.; Meyer, B.; Eckerlle, I.; Balavoine, J.-F.; Chappuis, F.; Pittet, D.; Trono, D.; et al. Persistence of anti-SARS-CoV-2 antibodies: Immunoassay heterogeneity and implications for serosurveillance. Clin. Microbiol. Infect. 2021, 27, 1695.e7–1695.e12. [Google Scholar] [CrossRef]
- Dowlatshahi, S.; Shabani, E.; Abdekhodaie, M.J. Serological assays and host antibody detection in coronavirus-related disease diagnosis. Arch. Virol. 2021, 166, 715–731. [Google Scholar] [CrossRef]
- Dai, L.; Gao, G.F. Viral targets for vaccines against COVID-19. Nat. Rev. Immunol. 2021, 21, 73–82. [Google Scholar] [CrossRef]
- Mesquita Júnior, D.; Araújo, J.A.P.; Catelan, T.T.T.; Souza, A.W.S.; Cruvinel, W.M.; Andrade, L.E.C.; Silva, N.P. Immune System Part II—Basis of the immunological response mediated by T and B lymphocytes. Brazilian J. Rheumatol. 2010, 50, 552–580. [Google Scholar] [CrossRef]
- Cancrini, G.; Iori, A. Traditional and innovative diagnostic tools: When and why should be applied. Parassitologia 2004, 46, 173–176. [Google Scholar] [PubMed]
- Ilkhani, H.; Hedayat, N.; Farhad, S. Novel approaches for rapid detection of COVID-19 during the pandemic: A review. Anal. Biochem. 2021, 634, 114362. [Google Scholar] [CrossRef]
- Matsuda, E.M.; de Campos, I.B.; de Oliveira, I.P.; Colpas, D.R.; dos Santos Carmo, A.M.; Brígido, L.F.M. Field evaluation of COVID-19 antigen tests versus RNA based detection: Potential lower sensitivity compensated by immediate results, technical simplicity, and low cost. J. Med. Virol. 2021, 93, 4405–4410. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Al Asoom, L.I.; Khan, M.; Chakrabartty, I.; Dandoti, S.; Rudrapal, M.; Zothantluanga, J.H. Evolution of RNA viruses from SARS to SARS-CoV-2 and diagnostic techniques for COVID-19: A review. J. Basic Appl. Sci. 2021, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Lumley, S.F.; Wei, J.; O’Donnel, D.; Stoesser, N.E.; Matthews, P.C.; Howarth, A.; Hatch, S.B.; Marsden, B.D.; Cox, S.; James, T.; et al. The Duration, Dynamics, and Determinants of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Antibody Responses in Individual Healthcare Workers. Clin. Infect. Dis. 2021, 73, e699–e709. [Google Scholar] [CrossRef] [PubMed]
- Villalta, D.; Moratto, A.; Salgarolo, V.; Da Re, M.; Giacomello, R.; Malipiero, G. New-Generation Quantitative Immunoassays for SARS-CoV-2 Antibody Detection: Need for Harmonization. Ann. Lab. Med. 2022, 42, 113–116. [Google Scholar] [CrossRef]
- Abusrewill, Z.; Alhudiri, I.M.; Kaal, H.H.; El Meshri, S.E.; Ebrahim, F.O.; Dalyoum, T.; Efrefer, A.A.; Ibrahim, K.; Elfghi, M.B.; Abusrewill, S.; et al. Time scale performance of rapid antigen testing for SARS-CoV-2: Evaluation of 10 rapid antigen assays. J. Med. Virol. 2021, 93, 6512–6518. [Google Scholar] [CrossRef]
- Girt, G.C.; Lakshminarayanan, A.; Huo, J.; Dormon, J.; Norman, C.; Afrough, B.; Harding, A.; James, W.; Owens, R.J.; Naismith, J.H. The use of nanobodies in a sensitive ELISA test for SARS-CoV-2 Spike 1 protein. R. Soc. Open Sci. 2021, 8, 211016. [Google Scholar] [CrossRef]
- Van der Moeren, N.; Zwart, V.F.; Goderski, G.; Rijkers, G.T.; van den Bijllaardt, W.; Veenemans, J.; Kluytmans, J.A.J.W.; Pas, S.D.; Meijer, A.; Verweij, J.J.; et al. Performance of the Diasorin SARS-CoV-2 antigen detection assay on the LIAISON XL. J. Clin. Virol. 2021, 141, 104909. [Google Scholar] [CrossRef]
- Amanat, F.; Thapa, M.; Lei, T.; Ahmed, S.M.S.; Adelsberg, D.C.; Carreño, J.M.; Strohmeier, S.; Schmitz, A.J.; Zafar, S.; Zhou, J.Q.; et al. SARS-CoV-2 mRNA vaccination induces functionally diverse antibodies to NTD, RBD, and S2. Cell 2021, 184, 3936–3948. [Google Scholar] [CrossRef]
- Krut, V.G.; Astrakhantseva, I.V.; Chuvpilo, S.A.; Efimov, G.A.; Ambaryan, S.G.; Drutskaya, M.S.; Nedospasov, S.A. Antibodies to the N-Terminal Domain of Angiotensin-Converting Enzyme (ACE2) That Block Its Interaction with SARS-CoV-2 S Protein. Dokl. Biochem. Biophys. 2021, 1–4. [Google Scholar] [CrossRef]
- Amjadi, M.F.; Adyniec, R.R.; Gupta, S.; Bashar, S.J.; Mergaert, A.M.; Braun, K.M.; Moreno, G.K.; O’Connor, D.H.; Friedrich, T.C.; Safdar, N.; et al. Anti-membrane and anti-spike antibodies are long-lasting and together discriminate between past COVID-19 infection and vaccination. medRxiv 2021. [Google Scholar] [CrossRef]
- Zhao, J.; Yuan, Q.; Wang, H.; Liu, W.; Liao, X.; Su, Y.; Wang, X.; Yuan, J.; Li, T.; Li, J.; et al. Antibody Responses to SARS-CoV-2 in Patients with Novel Coronavirus Disease 2019. Clin. Infect. Dis. 2020, 71, 2027–2034. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Ma, H.; Zeng, W.; He, H.; Zhao, D.; Jiang, D.; Zhou, P.; Cheng, L.; Li, Y.; Ma, X.; Jin, T. Serum IgA, IgM, and IgG responses in COVID-19. Cell. Mol. Immunol. 2020, 17, 773–775. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yu, X.; Gao, C.; Zhang, L.; Zhai, H.; Hu, Y.; Liu, E.; Wang, Q.; Gao, Y.; Wei, D.; et al. Characterization of antibody responses to SARS-CoV-2 in convalescent COVID-19 patients. J. Med. Virol. 2021, 93, 2227–2233. [Google Scholar] [CrossRef] [PubMed]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef]
- Swadzba, J.; Anyszek, T.; Panek, A.; Martin, E. Anti-Spike SARS-CoV-2 IgG Assessment with a Commercial Assay during a 4-Month Course after COVID-19 Vaccination. Vaccines 2021, 9, 1367. [Google Scholar] [CrossRef] [PubMed]
- Ciabattini, A.; Pastore, G.; Fiorino, F.; Polvere, J.; Lucchesi, S.; Pettini, E.; Stefano, A.; Rancan, I.; Durante, M.; Miscia, M.; et al. Evidence of SARS-CoV-2-Specific Memory B Cells Six Months After Vaccination With the BNT162b2 mRNA Vaccine. Front. Immunol. 2021, 12, 740708. [Google Scholar] [CrossRef]
- Atyeo, C.; Fischinger, S.; Zohar, T.; Slein, M.D.; Burke, J.; Loos, C.; McCulloch, D.J.; Newman, K.L.; Wolf, C.; Yu, J.; et al. Distinct Early Serological Signatures Track with SARS-CoV-2 Survival. Immunity 2020, 53, 524–532.E4. [Google Scholar] [CrossRef]
- Portilho, A.I.; Silva, V.O.; Ahagon, C.M.; Matsuda, E.M.; de Oliveira, E.L.; da Silveira, E.P.R.; Lima, A.K.S.; Lindoso, J.A.L.; Campos, I.B.; Hong, M.A.; et al. Humoral response to spike S1 and S2 and nucleocapsid proteins on microarray after SARS-CoV-2 infection. J. Med. Virol. 2021, 94, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.; Algaissi, A.; Almahboub, S.; Alfaleh, M.; Abujamel, T.; Alamri, S.; Alluhaybi, K.; Hobani, H.; AlHarbi, R.; Alsulaiman, R.; et al. Early Humoral Response Correlates with Disease Severity and Outcomes in COVID-19 Patients. Viruses 2020, 12, 1390. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Lucas, C.; Sundaram, M.; Israelow, B.; Wong, P.; Klein, J.; Lu, P.; Venkataraman, A.; Liu, F.; Mao, T.; et al. Saliva viral load is a dynamic unifying correlate of COVID-19 severity and mortality. medRxiv 2021. [Google Scholar] [CrossRef]
- Mestecky, J.; Mcghee, J.R. Immunoglobulin A (IgA): Molecular and Cellular Interactions Involved in IgA Biosynthesis and Immune Response. Adv. Immunol. 1987, 40, 153–245. [Google Scholar] [CrossRef]
- Kerr, M.A. The structure and function of human IgA. Biochem. J. 1990, 271, 285–296. [Google Scholar] [CrossRef]
- Hiemstra, P.S.; Gorter, A.; Stuurman, M.E.; van Es, L.A.; Daha, M.R. Activation of the alternative pathway of complement by human serum IgA. Adv. Exp. Med. Biol. 1987, 17, 321–326. [Google Scholar] [CrossRef]
- Sterlin, D.; Mathian, A.; Miyara, M.; Mohr, A.; Anna, F.; Claër, L.; Quentric, P.; Fadlallah, J.; Ghillani, P.; Gunn, C.; et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci. Transl. Med. 2021, 13, eabd2223. [Google Scholar] [CrossRef]
- Fedele, G.; Russo, G.; Schiavoni, I.; Leone, P.; Olivetta, E.; Perri, E.; Zingaropoli, M.A.; Ciardi, M.R.; Pasculli, P.; Mastroianni, C.M.; et al. Early IgG / IgA response in hospitalized COVID-19 patients is associated with a less severe disease. Diagn. Microbiol. Infect. Dis. 2022, 102, 115586. [Google Scholar] [CrossRef]
- Vossenkämper, A.; Blair, P.A.; Safinia, N.; Fraser, L.D.; Das, L.; Sanders, T.J.; Stagg, A.J.; Sanderson, J.D.; Taylor, K.; Chang, F.; et al. A role for gut-associated lymphoid tissue in shaping the human B cell repertoire. J. Exp. Med. 2013, 210, 1665–1674. [Google Scholar] [CrossRef]
- Victora, G.D.; Nussenzweig, M.C. Germinal Centers. Ann. Rev. Immunol. 2012, 30, 429–457. [Google Scholar] [CrossRef]
- Eisen, H.N. Affinity enhancement of antibodies: How low-affinity antibodies produced early in immune responses are followed by high-affinity antibodies later and in memory B-cell responses. Cancer Immunol. Res. 2014, 2, 381–392. [Google Scholar] [CrossRef]
- Correa, V.A.; Rodrigues, T.S.; Portilho, A.I.; Trzewikoswki de Lima, G.; De Gaspari, E. Modified ELISA for antibody avidity evaluation: The need for standardization. Biomed. J. 2021, 44, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Bauer, G. The potential significance of high avidity immunoglobulin G (IgG) for protective immunity towards SARS-CoV-2. Int. J. Infect. Dis. 2021, 106, 61–64. [Google Scholar] [CrossRef]
- Iwasaki, A.; Yang, Y. The potential danger of suboptimal antibody responses in COVID-19. Nat. Rev. Immunol. 2020, 20, 339–341. [Google Scholar] [CrossRef] [PubMed]
- Klasse, P.J.; Sattentau, Q.J. Occupancy and mechanism in antibody-mediated neutralization of animal viruses. J. Gen. Virol. 2002, 83, 2091–2108. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, J.; Li, Q.; Hu, H.; Lu, J.; Chen, Z. Advances in Neutralization Assays for SARS-CoV-2. Scand. J. Immunol. 2021, 94, 1–15. [Google Scholar] [CrossRef]
- Tan, C.W.; Chia, W.N.; Qin, X.; Liu, P.; Chen, M.I.C.; Tiu, C.; Hu, Z.; Chen, V.C.W.; Young, B.E.; Sia, W.R.; et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2–spike protein–protein interaction. Nat. Biotechnol. 2020, 38, 1073–1078. [Google Scholar] [CrossRef]
- Yin, Q.; Zhang, Y.; Lian, L.; Qu, Y.; Wu, W.; Chen, Z.; Pei, R.; Chen, T.; Sun, L.; Li, C.; et al. Chemiluminescence Immunoassay Based Serological Immunoassays for Detection of SARS-CoV-2 Neutralizing Antibodies in COVID-19 Convalescent Patients and Vaccinated Population. Viruses 2021, 13, 1508. [Google Scholar] [CrossRef]
- Neumann, F.; Rose, R.; Römpke, J.; Grobe, O.; Lorentz, T.; Fickenscher, H.; Krumbholz, A. Development of sars-cov-2 specific IgG and virus-neutralizing antibodies after infection with variants of concern or vaccination. Vaccines 2021, 9, 700. [Google Scholar] [CrossRef]
- von Rhein, C.; Scholz, T.; Henss, L.; Kronstein-Wiedemann, R.; Schwarz, T.; Rodionov, R.N.; Corman, V.M.; Tonn, T.; Schnierle, B.S. Comparison of potency assays to assess SARS-CoV-2 neutralizing antibody capacity in COVID-19 convalescent plasma. J. Virol. Methods 2021, 288, 114031. [Google Scholar] [CrossRef]
- Meyer, B.; Reimerink, J.; Torriani, G.; Brouwer, F.; Godeke, G.J.; Yerly, S.; Hoogerwerf, M.; Vuilleumier, N.; Kaiser, L.; Eckerle, I.; et al. Validation and clinical evaluation of a SARS-CoV-2 surrogate virus neutralisation test (sVNT). Emerg. Microbes Infect. 2020, 9, 2394–2403. [Google Scholar] [CrossRef] [PubMed]
- Cruvinel, W.M.; Júnior, D.M.; Araújo, J.A.P.; Catelan, T.T.T.; de Souza, A.W.S.; da Silva, N.P.; Andrade, L.E.C. Immune system—Part I fundamentals of innate immunity with emphasis on molecular and cellular mechanisms of inflammatory response. Rev. Bras. Reumatol. 2010, 50, 443–461. [Google Scholar] [CrossRef]
- Hogrefe, W.R. Biomarkers and assessment of vaccine responses. Biomarkers 2005, 10 (Suppl. 1), 50–57. [Google Scholar] [CrossRef] [PubMed]
- Freer, G.; Rindi, L. Intracellular cytokine detection by fluorescence-activated flow cytometry: Basic principles and recent advances. Methods 2013, 61, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Czerkinsky, C.C.; Svennerholm, M.S. Ganglioside GM1 enzyme-linked immunospot assay for simple identification of heat-labile enterotoxin-producing Escherichia coli. J. Clin. Microbiol. 1983, 17, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Czerkinsky, C.C.; Nilsson, L.A.; Nygren, H.; Ouchterlony, O.; Tarkowski, A. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J. Immunol. Methods 1983, 65, 109–121. [Google Scholar] [CrossRef]
- Janetzki, S. ELISpot for Rookies (and Experts Too), 1st ed.; Springer Nature: Cham, Switzerland, 2016; ISBN 978-3-319-45293-7. [Google Scholar]
- Lima-Junior, J.; Morgado, F.; Conceição-Silva, F. How Can Elispot Add Information to Improve Knowledge on Tropical Diseases? Cells 2017, 6, 31. [Google Scholar] [CrossRef]
- Tripathy, A.S.; Vishwakarma, S.; Trimbake, D.; Gurav, Y.K.; Potdar, V.A.; Mokashi, N.D.; Patsute, S.D.; Kaushal, H.; Choudhary, M.L.; Tilekar, B.N.; et al. Pro-inflammatory CXCL-10, TNF-α, IL-1β, and IL-6: Biomarkers of SARS-CoV-2 infection. Arch. Virol. 2021, 166, 3301–3310. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.; Huang, F.; Yang, Y.; Wang, F.; Yuan, J.; Zhang, Z.; Qin, Y.; Li, X.; Zhao, D.; et al. Elevated plasma levels of selective cytokines in COVID-19 patients reflect viral load and lung injury. Nat. Sci. Rev. 2020, 7, 1003–1011. [Google Scholar] [CrossRef]
- Bösmüller, H.; Traxler, S.; Bitzer, M.; Häberle, H.; Raiser, W.; Nann, D.; Frauenfeld, L.; Vogelsberg, A.; Klingel, K.; Fend, F.; et al. The evolution of pulmonary pathology in fatal COVID-19 disease: An autopsy study with clinical correlation. Virchows Arch. 2020, 477, 349–357. [Google Scholar] [CrossRef]
- Hassaniazad, M.; Vahedi, M.S.; Samimagham, H.R.; Gharibzadeh, A.; Beyranvand, S.; Abassi, H.; Nikpoor, A.R. Improvement of clinical outcome, laboratory findings and inflammatory cytokines levels using plasmapheresis therapy in severe COVID-19 cases. Respir. Med. 2021, 189, 106669. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020, 26, 1636–1646. [Google Scholar] [CrossRef] [PubMed]
- Angioni, R.; Sánchez-Rodriguéz, R.; Munari, F.; Bertoldi, N.; Arcidiacono, D.; Cavinato, S.; Marturano, D.; Zaramella, A.; Realdon, S.; Cattelan, A.; et al. Age-severity matched cytokine profiling reveals specific signatures in Covid-19 patients. Cell Death Res. 2020, 11, 957. [Google Scholar] [CrossRef] [PubMed]
- Batista, C.M.; Foti, L. Anti-SARS-CoV-2 and anti-cytokine storm neutralizing antibody therapies against COVID-19: Update, challenges, and perspectives. Int. Immunopharmacol. 2021, 99, 108036. [Google Scholar] [CrossRef] [PubMed]
- Mortaz, E.; Tabarsi, P.; Varahram, M.; Folkerts, G.; Adcock, I.M. The Immune Response and Immunopathology of COVID-19. Front. Immunol. 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Li, G.; Fan, Y.; Lai, Y.; Han, T.; Li, Z.; Zhou, P.; Pan, P.; Wang, W.; Hu, D.; Liu, X.; et al. Coronavirus infections and immune responses. J. Med. Virol. 2020, 92, 424–432. [Google Scholar] [CrossRef]
- Foulds, K.E.; Wu, C.Y.; Seder, R.A. Th1 memory: Implications for vaccine development. Immunol. Rev. 2006, 211, 58–66. [Google Scholar] [CrossRef]
- Schwarzkopf, S.; Krawczyk, A.; Knop, D.; Klump, H.; Heinold, A.; Heinemann, F.M.; Thümmler, L.; Temme, C.; Breyer, M.; Witzke, O.; et al. Cellular immunity in COVID-19 convalescents with PCR-confirmed infection but with undetectable SARS-CoV-2-specific IgG. Emerg. Infect. Dis. 2021, 27, 122–129. [Google Scholar] [CrossRef]
- Li, J.; Hui, A.; Zhang, X.; Yang, Y.; Tang, R.; Ye, H.; Ji, R.; Lin, M.; Zhu, Z.; Türeci, Ö.; et al. Safety and immunogenicity of the SARS-CoV-2 BNT162b1 mRNA vaccine in younger and older Chinese adults: A randomized, placebo-controlled, double-blind phase 1 study. Nat. Med. 2021, 27, 1062–1070. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Stephenson, K.E.; Le Gars, M.; Sadoff, J.; De Groot, A.M.; Heerwegh, D.; Truyers, C.; Atyeo, C.; Loos, C.; Chandrashekar, A.; McMahan, K.; et al. Immunogenicity of the Ad26.COV2.S Vaccine for COVID-19. JAMA—J. Am. Med. Assoc. 2021, 325, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.; Tascilar, K.; Schmidt, K.; Manger, B.; Weckwerth, L.; Sokolova, M.; Bucci, L.; Fagni, F.; Manger, K.; Schuch, F.; et al. Brief Report: Humoral and cellular immune responses to SARS-CoV-2 infection and vaccination in B cell depleted autoimmune patients. Arthritis Rheumatol. 2022, 74, 33–37. [Google Scholar] [CrossRef]
- Krammer, F. A correlate of protection for SARS-CoV-2 vaccines is urgently needed. Nat. Med. 2021, 27, 1145–1146. [Google Scholar] [CrossRef] [PubMed]
- Bauer, G.; Struck, F.; Schreiner, P.; Staschik, E.; Soutschek, E.; Motz, M. The challenge of avidity determination in SARS-CoV-2 serology. J. Med. Virol. 2021, 93, 3092–3104. [Google Scholar] [CrossRef] [PubMed]
- Struck, F.; Schreiner, P.; Staschik, E.; Wochinz-Richter, K.; Schulz, S.; Soutschek, E.; Motz, M.; Bauer, G. Vaccination versus infection with SARS-CoV-2: Establishment of a high avidity IgG response versus incomplete avidity maturation. J. Med. Virol. 2021, 93, 6765–6777. [Google Scholar] [CrossRef] [PubMed]
- Pratesi, F.; Caruso, T.; Testa, D.; Tarpanelli, T.; Gentili, A.; Gioè, D.; Migliorini, P. Bnt162b2 mrna sars-cov-2 vaccine elicits high avidity and neutralizing antibodies in healthcare workers. Vaccines 2021, 9, 672. [Google Scholar] [CrossRef]
- Taylor, S.C.; Hurst, B.; Martiszus, I.; Hausman, M.S.; Sarwat, S.; Schapiro, J.M.; Rowell, S.; Lituev, A. Semi-quantitative, high throughput analysis of SARS-CoV-2 neutralizing antibodies: Measuring the level and duration of immune response antibodies post infection/vaccination. Vaccine 2021, 39, 5688–5698. [Google Scholar] [CrossRef]
This entry is adapted from the peer-reviewed paper 10.3390/jcm11061503
