Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Medicine, Research & Experimental
Bergenin (BER), a key constituent of Bergenia crassifolia (Saxifragaceae), has gained extensive attention, owing to its array of pharmacological actions, including anti-infective, anti-cancer, anti-diabetic, neuroprotective, hepatoprotective, anti-urolithiatic, anti-hyperuricemic, and anti-bradykinin properties.
- Bergenia crassifolia
- anti-inflammatory
- novel carriers
- antioxidant
- bioactive
1. Introduction
With improvements in public awareness, attempts have been continuously made to explore safer alternative remedies for various health issues. Furthermore, the toxic effects of chemically derived drugs and irrepressible risks linked with biological products strengthen the need for an investigation of naturally derived compounds [1]. Nature is a rich source of extremely innovative and diverse bioactive compounds [2]. Plants are incredible in their potential to generate a huge number of specialized metabolites and byproducts with different biological actions. Natural constituents have been used as models for the development of drugs [3] and provided unquestionable support for human welfare [4]. Bergenin (BER) is a natural constituent, which has been extracted from various parts (rhizome, roots, leaves, stem, barks, aerials, seeds, cortex, flowers, wood, tuber, heartwood, fruit or whole plant) of plants [5] such as Bergenia crassifolia (B. crassifolia), Bergenia ciliata (Saxifragaceae), Corylopsis spicata (C. spicata), Mallotus philippinensis (M. philippinensis), Caesalpinia digyna (C. digyna), Sacoglottis gabonensis (S. gabonensis), and Mallotus japonicus (M. japonicus) (Table 1 and Figure 1). It is commonly called Pashaanbheda (Paashan; rockstone, bheda; piercing) and Zakham-e-hayat (zakham; lesion/wound, hayat; life/heal) in the Indian Systems of Medicine [6,7]. It is officially listed in the People’s Republic of China (Chinese Pharmacopoeia Commission, 2010) [8]. In accordance with a citation in the Merck Index, this bioactive compound was firstly isolated from Saxifraga (Bergenia) siberica rhizomes [5,9]. BER is trihydroxybenzoic acid glycoside [10]. Traditionally, the rhizomes of Bergenia have been used for the treatment of fractured bones, wounds, fresh cuts, pulmonary infections, diarrhea, vomiting, cough, boils, and fever by local people [11,12]. The roots of Bergenia have been employed as a deobstruent, demulcent, reliever for ribs and chest pain, a emmenagogue, and a diuretic [6]. The virtues of plants are attributed, to a large extent, to the formation of their secondary metabolites, including bergenin, catechin, and gallic acid, which are mainly used in traditional drugs [13]. BER is a versatile phytoconstituent, as it holds numerous beneficial pharmacological characteristics such as heart disorders, stomach diseases, hemorrhoids, and ophthalmia treatment [2,9]. Additionally, it is accredited with anti-viral, analgesic, antimalarial, antioxidant, and anti-inflammatory potential [7,14]. Owing to these properties, its use as a natural alternative to cure various ailments has increased dramatically in the past decade. Despite the fact that it possesses a wide array of activities, the inherent physicochemical properties of BER limit its pharmaceutical use. The major limitations allied with its delivery are low solubility and poor permeability. Neither highly hydrophilic nor highly lipophilic BER possess poor oral bioavailability. It is commercially available as tablets, pills, and soft gelatin capsules [15] (Table 2, Figure 2), however, the efficacy of these traditional formulations of BER is far lower than expectations [8]. Therefore, novel delivery systems may prove to be promising for overcoming the inherent constraints of BER. It is well known that novel carriers possess a profound potential to improve solubility and stability, modify release behavior, and consequently, enhance the efficacy of entrapped moieties. A handful of BER formulations reported in the literature encompass phospholipid complexes, extended-release core tablets, prodrugs, herbal gels, poly herbal ointment, nanoparticles, and poly (lactic acid) polymers. There is a large number of research and review articles that mainly focus on the role of novel delivery carriers in surpassing the issues associated with bioactive compounds. The available information about BER was collected from popular and widely used databases, such as Web of Science, Google Scholar, Scopus, PubMed, Science Direct, and Springer search. From these searches, a number of citations related to the pharmacological activities, novel formulations, pharmacokinetics, applications, chemistry and physicochemical properties of phytoconstituent BER were retrieved. The keywords used include pharmacological activities, novel carriers, pharmacokinetics, chemistry, patents, and other related words [7].
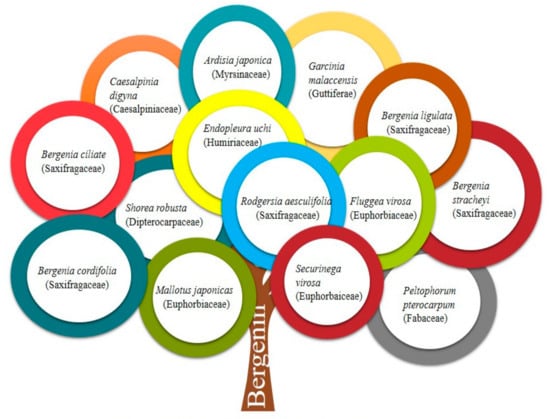
Figure 1. Various plant sources of Bergenin.
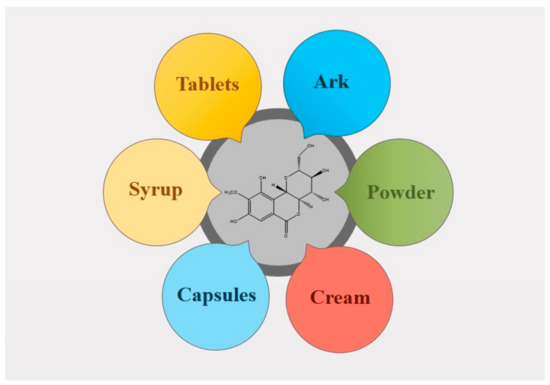
Figure 2. Conventional formulations of Bergenin.
Table 1. Pharmacological/biological activities of various plants rich in Bergenin.
| Sr. No. | Plants (Families) | Part Used | Pharmacological/Biological Activities | Mechanism(s) of Action | Study Models | References |
|---|---|---|---|---|---|---|
| 1. | Bergenia ligulata | Rhizome | Anti-microbial | Inhibits anaerobic glycosis and aerobic respiration | Agar well-diffusion assay | [16] |
| 2. | Bergenia spp. | - | Anti-cancer (cervical cancer) |
Inaugural of apoptosis and cell cycle inhibition in the G0/G1 phase. Inhibit phosphorylation of STAT3 proteins. |
Cervical cancer cell line HeLa | [17] |
| 3. | Bergenia ciliata, Bergenia spp. Bergenia stracheyi |
Aerial parts - Rhizome |
Anti-inflammatory, immunomodulatory | Inhibition of IL-6 and TNF-α; Targeting cytokine (IL-1b, IL-6 and TNF-α) and reactive oxygen species (ROS), Prevent the development of proinflammatory Th1 cytokines (IFN-γ, IL-2 and TNF-α) whereas potentiate anti-inflammatory Th2 cytokines (IL-5 and IL-4) | Human monocyte leukemia THP-1 cells, Carrageenan-induced paw edema and Mycobacterium-induced arthritis in rats; CFA-induced arthritis model |
[18,19,20] |
| 4. | Bergenia stracheyi, Bergenia ligulata, Bergenia cordifolia, Endopleura uchi, Peltophorum pterocarpum |
Rhizome Bark, Flowers |
Antioxidant | Free radical scavenging activity | DPPH assay, Agar well diffusion method, Disc diffusion method | [2,21,22] |
| 5. | Bergenia cordifolia, Caesalpinia digyna Rottler |
Rhizome Roots |
Anti-diabetic | Inhibition of α-glucosidase enzyme. Positive effect on endocrine cells of pancreas results in enhanced development of insulin. |
Microtitre-based assay. Streptozotocin-nicotinamide induced diabetic rats. |
[22,23] |
| 6. | Mallotus japonicus | Dried bark | Neuroprotective | Inhibit generation of ROS in brain | Culture of rat cortical neurons in DMEM supplemented with Nitrogen | [24] |
| 7. | Mallotus japonicas | Cortex | Hepatoprotective | Attenuated the increase in the activities of alanine aminotransferase, sorbitol dehydrogenase, aspartate aminotransferase, γ-glutamyltransferase and also inhibit lipid peroxidation and recover the reduced hepatic glutathione level | CCl4-induced hepatic damage in rats | [25] |
| 8. | Mallotus philippinensis | Leaf | Anti-urolithiatic | Significantly reduction in calcium, oxalate and phosphate concentration in urine | Ethylene glycol-induced urolithiasis in wistar rats | [26] |
| 9. | Rodgersia aesculifolia Batal, Bergenia ligulata | Rhizome | Anti-malarial | Inhibition of heme polymerization pathway of malaria parasite | In vitro and In vivo assessment of antimalarial activity using Plasmodium falciparum and Plasmodium berghei infected BALB/c mice | [7,21,27] |
| 10. | Caesalpinia digyna Rottler | Root | Anxiolytic | - | EPM (mice) | [7] |
| 11. | Shorea robusta | Leaves | Anti-tubercular | Induces the production of TNF-α, NO, IFN-γ, IL-17 and IL-12 from both CD4 and CD8 T-cells | Murine model of Mycobacterim tuberculosis infection | [28] |
| 12. | Bergenia stracheyi | Rhizome | Anti-gout | Inhibition of xanthine oxidase enzyme | Assayed spectrophotometrically | [2] |
| 13. | Garcinia malaccensis | Stembark | Antiplatelet aggregation | Inhibition of platelet aggregation induced by arachidonic acid, adenosine diphosphate and collagen | Platelet aggregation test measured by ANOVA | [3] |
| 14. | Flueggea microcarpa | Leaves | Antihyperlipidemic | Reduced level of CH, LDL, VLDL, TG and increased proportion of HDL were reported via, increasing reverse cholesterol transport from arterial tissue to the liver | Albino rats of Charles Foster strain given hyperlipidaemic diet of arachis oil | [29] |
| 15. | Ardisia japonica | Aerial parts | Anti-HIV | Inhibition of antibody ADP358 binding to gp120 and interfere with gp120-CD4 interaction | C8166 cells infected with HIV-1 |
[30] |
| 16. | Fluggea virosa | Aerial parts | Anti-arrhythmic | Coronary artery ligation and blood reperfusion | BaCl2 induced arrhythmia in rats | [31] |
| 17. | Flueggea microcarpa | Leaves and roots | Antiulcer | Protection against pylorus-ligated and gastric ulcers induced by aspirin | Gastric ulcers induced by cold restraint stress-induced in guinea pigs and rats. | [32] |
| 18. | Securinega virosa | Root bark | Soporific | - | Beam walking test and Diazepam-induced sleeping time assay in mice. | [33] |
| 19. | Bergenia ciliata | Rhizome | Anti-tussive | Bronchodilator action, inhibited the histamine and acetylcholine induced contractions | Cough model induced by sulphur dioxide gas in mice | [34,35] |
Table 2. Indian conventional formulations for Bergenin.
| Sr. No. | Commercial Herbal Formulations | Name and Amount of Extract Containing Bergenin | Therapeutic Dose Required | Potential Uses/Indications | Manufacturers |
|---|---|---|---|---|---|
| 1. | Albestone Capsule | Pashanbhed (Bergenia ligulata) 200 mg | As per directed by doctor | Kidney calculi, Bladder calculi, Ureter calculi, Retention of urine, Calculi induced UTI | Sanify Healthcare Pvt. Ltd. (Punjab) |
| 2. | Phytone Capsule | Pashanbhed Extract (Saxifrage lingulate) 100 mg |
1–2 Capsules twice daily | Medical Management of Urinary Calculi, for the prevention of recurrent calculi. | Abhinav Healthcare |
| 3. | Stonvil Capsule | Pashanbhed, (Saxifraga ligulata) 30 mg | U. T. I.: 2 b. d. for 2 weeks. Renal calculi: 2 b.d. upto 3 weeks. Burning Micturition: 1 b.d. upto 2 weeks. |
Burning micturition, Grit, Calculi problems and Urinary tract infections | S.G Phyto Pharma Pvt. Ltd. |
| 4. | Cystone Tablet | Saxifraga ligulata (98 mg/tab.) | 2 Tabs. twice daily | Gritty kidney, Ureter, bladder and urethra Sialolithiasi, Urinary tract infection (UTI), Colic ureter, Glomerulonephritis crystalluria—fosfatouria Heart-Renal Edema, Bed wetting-urinary incontinence, Hyperuricemia Enlarged prostate: in concomitant use with speman or Himplasia prevents surgery. | The HimalayaTM Drug Company |
| 5. | Nefrotec~ DS VET Tablet | Pashanbhed (Saxifraga ligulata 30 mg) | Dogs: 1 Tablet two times daily for small breeds. 2 Tablets two times daily for large breeds Cats: 1 Tablet one time a day. |
Nephrolithiasis, Recurrent urinary tract infections, Cystitis, Non-specific Urethritis, kidney dysfunction. | The HimalayaTM Drug Company |
| 6. | Neeri Tablet | Bergenia ligulata (60 mg/tab.) | Children: 1–2 Tabs. twice a day. Adults: 2–3 Tabs. thrice a day. |
Dysuria, Burning Micturition, Crystalluria, Oedema, Anasarca, Non-specific UTIs. | Aimil Pharmaceuticals Ltd. |
| 7. | Patharina Tablet | Pashanbhed - |
2 Tablets twice a day orally with water or as directed by the physician. | Kidney Stones, Painful Urination | Shree Baidyanath Ayurved Bhawan Pvt. Ltd. |
| 8. | Cystone Syrup | Saxifraga ligulata (53 mg/5 mL) | Children: ½-1 Teaspoonful (2.5–5 mL) twice daily after meals. Adults: 1–2 Teaspoonful (5–10 mL) twice daily after meals. |
Kidney stones, Crystalluria, crystals in the urine, Dysuria, Hyperuricemia, high amount of uric acid in the blood, Burning while urination, Non-specific Urethritis, i.e., irritation or swelling of the urethra. |
The HimalayaTM Drug Company |
| 9. | Neeri Syrup | Bergenia ligulata (100 mg/10 mL) | Children: ½–1 Teaspoonful thrice daily. Adults: 2 teaspoonful thrice daily. |
Dysuria, Burning Micturition, Oedema, Anasarca, Non-specific UTIs. | Aimil Pharmaceuticals Ltd. |
| 10. | StonDab Syrup | Pashanbheda - |
1–2 Teaspoonful of the syrup 3 times a day. | kidney stones, burning sensation while urination, non-specific urinary tract infection, urinary calculus | Dabar India limted |
| 11. | Ashmarihar kwath Powder | Saxifraga ligulata (15 g/100 g) | Mix 5–10 gm of kwath in around 400 mL water and boil it, till residue is 100 mL. | Kidney stone, gall stone problem. | Divya Pharmacy |
| 12. | Pashan Bhed Root Powder | (Saxifraga ligulata powder roots) 3 mg | 1–2 Tablespoon mix with water, blend in a smoothie drink/sprinkle over salad. | Urinary tract infection (UTI) burning and painful micturition, spleen related swelling. | Bixa botanical |
| 13. | Pushyanug Churna | (Saxifraga ligulata) 5 mg | 2–3 mg, twice a day. | leucorrhoea, menorrhagia, metrorrhagia, prolapse of uterus and also useful in diarrhea, dysentery and bleeding piles | Deep Ayurveda |
| 14. | Prakriti Pashanbhed Ark | Pashanbhed - |
10–15 mL of Pashanbhed ark, Twice a day with equal amount of warm water before meals. | Kidney Stones and Liver related problems | Prakriti Nutann Gausadan |
| 15. | Pashan Bhed transdermal Cream | Pashanbhed - |
Whole spine Swiping downwards 7 times in the morning and evening. | Autoimmune toxins, gall stone, inflammation, kidney stones | Prabhava Ayurvedic herbals |
2. Chemical Structure & Physicochemical Properties of BER
BER [IUPAC Name: 3,4,8,10-tetrahydroxy-2-(hydroxymethyl)-9-methoxy 3,4,4a,10b-tetrahydro-2H-pyranol [3,2-c]isochromen-6-one; molar mass: 328.27 gmol−1 and molecular formula: C14H16O9] is [15] a white, crystalline powder or loose needle-like crystal powder with a bitter taste and light odor. It becomes discolored upon exposure to heat or light. It has a melting point and a specific optical rotation in the range 232–240 °C and −38° to −45°, respectively [36]. Log p-values (−1.060 ± 0.033 to −1.19 ± 0.044) at temperature 37 °C and acidic pH confined the poor lipophilic property of this moiety [37]. It is a C-glucoside of 4-O-methyl gallic acid (2β-D-glucopyranosyl 4-O-methylgallic acid δ lactone) [5]. The initial structures of this molecule were given in 1928 by Tschitschibabin et al. [38] structure I, and Shimokôriyama, structure II, in 1950. These structures were revised by Hay et al. [39], Posternak et al. [40] and Fujise et al. in the year 1959 [41] [Figure 3a]. The conformation of this moiety was unequivocally established by an X-ray analysis of its monohydrate and 3, 4, 8, 10, 11- penta acetate derivatives [42].
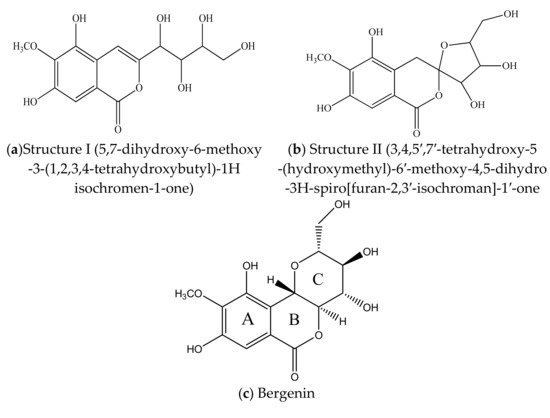
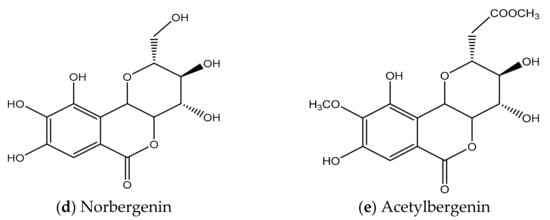
Figure 3. (a,b). Initial structures of Bergenin. (c–e) Structure of Bergenin and their analogs.
The molecule BER is comprised of three six-membered rings: (A) an aromatic ring, (B) an annellated δ-lactone ring, and (C) a glucopyranose ring. The ring (C) is only slightly different to an ideal chair structure. The ring (B) exhibits the predictable half-chair conformation. There are six inter- and one intra-molecular hydrogen bonds that outline an extensive hydrogen-bonding arrangement within the crystal. Figure 3b represents the chemical structure of BER and its two analogues—acetylbergenin and norbergenin [43]. Furthermore, Ye et al. have demonstrated that there are six inter- and one intra-molecular hydrogen bond, outlining an extensive hydrogen-bonding net within the crystal, thus it does not have a great number of energetic (active) sites for water sorption [44].
BER possess low aqueous solubility, which results in its poor oral bioavailability. Its poor permeability and low solubility are the major obstacles in its formulation development. Commercially, it is available as soft gelatin capsules, tablets, and pills (Table 2 and Figure 2). Liquid dosage forms for BER are not available on the market, owing to its poor aqueous solubility. The solubility of BER was reported to be highest in polyethylene glycol-400 (PEG-400), followed by dimethyl sulfoxide (DMSO), diethylene glycol monoethyl ether, propylene glycol (PG), ethylene glycol (EG), ethanol, isopropanol (IPA), ethyl acetate (EA), 2-butanol, 1-butanol, and water in a range of temperatures (298.15 to 318.15 K) and pressures 0.1 MPa [15]. Generally, the low water uptake of BER suggested good stability in the presence of moisture during formulation and storage [37].
3. Mode of Action of BER
Free radicals are very active molecules that are formed through normal metabolism and cellular respiration. Reactive oxygen species (ROS) are intimately associated with pathological and physiological processes in animals. Chiefly, these species are hydrogen peroxide (H2O2), hydroxyl free radicals (OH−), superoxide anion free radicals (O2−), nitrogen oxide radicals (NO−), and others. At lower levels, ROS can work as signaling molecules which control basic cellular mechanisms, such as cellular adaptive and cell growth responses [45]. The excess formation of such free radicals can lead to oxidative injury to biomolecules (proteins, DNA, lipids) [46]. Furthermore, due to imbalances between the body’s antioxidant process and the accumulation of ROS, oxidative stress occurs which damages tissues and cells, resulting in the proliferation of numerous ailments. There are clear facts that free radicals are connected with the propagation of ailments, like cancer, atherosclerosis, and emphysema [45].
Bergenin (and its congeners) are extensively employed in Ayurvedic, Traditional Chinese Medicine, Unani, and various folk systems of medicine [5,9]. This bioactive has gained noteworthy attention and is a medicine of choice, by virtue of its multi-target approaches. BER is known for its multiple pharmacological features; anti-inflammatory, anti-oxidative, anti-arthritic, and anti-cancer activities [47], as in Table 1 and Figure 4. In addition, some of the patents related to BER activities are listed in Table 3. The effectiveness of this phytochemical participates in several mechanisms, such as lipid peroxidation inhibitory activity, free radical scavenging activity [6], initiating apoptosis and cell cycle arrest in the G0/G1 phase, inhibiting the phosphorylation of STAT3 proteins, inducing the formation of TNF-α, NO, IFN-γ, IL-17, IL-12, and inhibiting the α-glucosidase enzyme (Table 1, Figure 5). All of these have been explained in detail in the sections below [43].
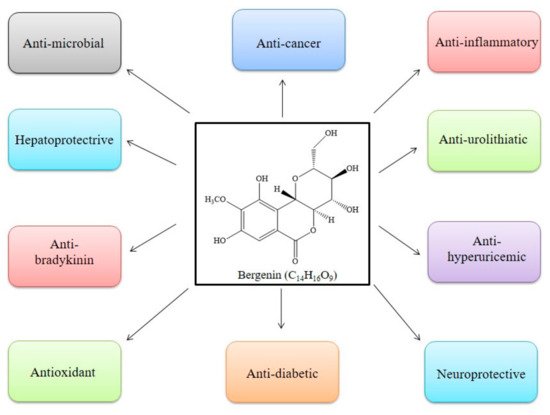
Figure 4. Pharmacological activities reported in literature for Bergenin.
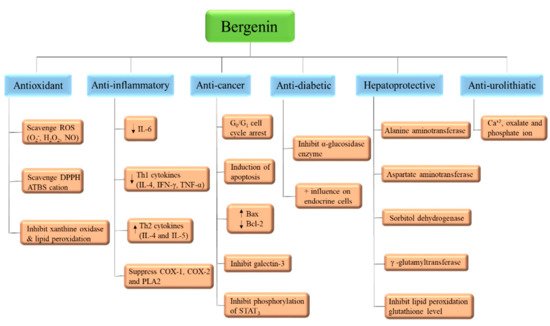
Figure 5. Mode of action of Bergenin.
Table 3. List of Bergenin Patents.
| Sr. No. | Patents No./Patent Publication No. | Title | Invention | References |
|---|---|---|---|---|
| 1. | US 10,494,377 B1 | Bergenin lipoic acid ester with antioxidant activity and a method of preparing the same | Invented BER lipoic acid ester having excellent antioxidant potential. | [48] |
| 2. | US 8,007,837 B2 | Herbal composition for maintaining/caring the skin around the eye, methods of preparing the same and uses thereof | Invented a novel herbal skinceutical composition to maintain and improve skin health especially for delicate skin around the eyes comprising the extracts of Saxifraga ligulata, Cipadessa baccifera and Emblica officinalis, method for preparing the same and use | [49] |
| 3. | US 7,785,637 B2 | Herbal composition for other publications maintaining/caring the skin around the eye, methods of preparing the same and uses thereof | Invented a novel herbal skinceutical composition to improve and maintain skin health especially for delicate skin around the eyes comprising the extracts of Saxifraga ligulata, Cipadessa baccifera and Emblica officinalis, method for preparing the same and their uses. | [50] |
| 4. | 217147 | A Pharmaceutical Composition Useful as an Antioxidant | Invented the process of isolation of BER from Tinospora crispa. | [51] |
| 5. | US 2004/O115286A1 | Cosmetic composition of Remedying skin wrinkles comprising bergenia Emeensis extract as active ingredient | Invented a cosmetic composition having Bergenia emeiensisextract, for skin wrinkles owing to its potential inhibition of collagenase and elastase. | [52] |
| 6. | WO2019077620A1 | Gastroretentive sustained release formulations of Bergenia ciliata | Invented novel gastroretentive swellable oral formulations for sustained or delayed release of BER-rich Bergenia ciliata extract/fraction and a process for preparing the same. The novel formulations were found to be retained in the stomach, which avoids intestinal degradation of BER resulting in its sustained release in stomach over a time period of 16–24 h. | [53] |
| 7. | US 10,494,377 B1 | Bergenin lipoic acid ester with antioxidant activity and a method of preparing the same | Invented BER lipoic acid ester having excellent antioxidant potential. | [48] |
| 8. | US 8,007,837 B2 | Herbal composition for maintaining/caring the skin around the eye, methods of preparing the same and uses thereof | Invented a novel herbal skinceutical composition to maintain and improve skin health especially for delicate skin around the eyes comprising the extracts of Saxifraga ligulata, Cipadessa baccifera and Emblica officinalis, method for preparing the same and use | [49] |
| 9. | US 7,785,637 B2 | Herbal composition for other publications maintaining/caring the skin around the eye, methods of preparing the same and uses thereof | Invented a novel herbal skinceutical composition to improve and maintain skin health especially for delicate skin around the eyes comprising the extracts of Saxifraga ligulata, Cipadessa baccifera and Emblica officinalis, method for preparing the same and their uses. | [50] |
| 10. | 217147 | A Pharmaceutical Composition Useful as an Antioxidant | Invented the process of isolation of BER from Tinospora crispa. | [51] |
| 11. | US 2004/O115286A1 | Cosmetic composition of Remedying skin wrinkles comprising bergenia Emeensis extract as active ingredient | Invented a cosmetic composition having Bergenia emeiensis extract, for skin wrinkles owing to its potential inhibition of collagenase and elastase. |
[52] |
| 12. | WO2019077620A1 | Gastroretentive sustained release formulations of Bergenia ciliata | Invented novel gastroretentive swellable oral formulations for sustained or delayed release of BER-rich Bergenia ciliata extract/fraction and a process for preparing the same. The novel formulations were found to be retained in the stomach, which avoids intestinal degradation of BER resulting in its sustained release in stomach over a time period of 16–24 h. | [53] |
4. Applications
Various applications of bergenin are elaborated previously in Table 1.
4.1. Anxiolytic
Anxiety ailment is the most common mental ailment faced by adolescents and children. Prevalence rates of anxiety are from 13.6 to 28.8% in western nations and 4.5% of the global population. The anti-anxiety potential of BER was examined by Singh and his co-workers. BER has been shown to have remarkable anti-anxiety potential (at 80 mg/kg, per oral), i.e., statistically similar to diazepam (2 mg/kg/per oral). BER exhibited significant anxiolytic potential in mirrored chamber and open field tests as well [74].
4.2. Antimalarial
Malaria is a severe protozoal parasitic disease transmitted by Anopheles mosquitoes (female) [27]. As per the literature evidence, BER is highly active both for chloroquine-sensitive (CQS) and chloroquine-resistant (CQR) P. falciparum. BER represents its action through generating oxidative stress and via preventing hemozoin formation, and therefore provokes the decease of malaria parasites [75].
Uddin et al. have assessed anti-plasmodial properties of BER in comparison to 11-O-galloylbergenin (its natural derivative). Both constituents were collected from Bergenia ligulata [21]. Liang and researchers also examined the antimalarial activity of BER (derived from Rodgersiaaes culifolia Batal). BER successfully prevented the in vitro growth of P. falciparum, besides apparent cytotoxic to mammalian HepG2 and HeLa cell line or to erythrocytes. BER administration to Plasmodium berghei-infected mice for 6 days remarkably prevented the extension of the parasites [27]. Singh and co-workers bioprospected leaves of Flueggeavirosa for its anti-malarial efficiency and active principles. BER showed modest anti-malarial action against P. berghei and reduced parasites causing systemic inflammation in mice as well [76]. In a nutshell, these outcomes substantiated that BER is a potential bioactive molecule for the management of malaria.
4.3. Antituberculosis
Tuberculosis is one of the most global health concerns, which has delayed socio-economic progress in various areas of the world [28].
Dwivedi and their research group showed that BER (from tender leaves of Shorearobusta) activates ERK and MAP kinase pathways and stimulates NO, IL-12 and TNF-α formation in infected types of macrophages. Furthermore, BER stimulates Th1 immune signals and potentially prevents bacillary growth in a mycobacterium tuberculosis-infected murine model. These findings identified BER as a potential bioactive for TB management [28].
Kumar and his research team found that this bioactive candidate stimulates T helper 17 (Th17)- and Th1 cellular defensive immune responses, and potentially prevents the growth of mycobacterials in the mycobacterium tuberculosis-infected murine model. Of note, BER treatment remarkably declined the bacterial burden of an MDR TB strain. These outcomes demonstrated that BER is a powerful immunomodulatory drug candidate which can be explored as a prospective adjunct to TB treatment in near future [77].
4.4. Antiplatelet Aggregation
Thrombosis is the biggest cause of death in the world. This disorder is intimately associated to a chain of cascades such as secretory, aggregation and adhesive purposes of the activated platelet, and the activation of the extrinsic and intrinsic coagulation systems, which are accountable for fibrin formation and blood coagulation. In particular, the aggregation of platelets significantly participated in several thromboembolic ailments as well [78].
Alkadi and his research team isolated four known constituents, 5-Hydroxyflavone, 2′-Hydroxyflavanone, Paeonol, and BER, from the stem bark of Garciniamalaccensis. All isolated components were found to exhibit the prevention of platelet aggregation in human blood, stimulated by collagen, ADP (adenosine diphosphate), and AA (arachidonic acid) [3].
This entry is adapted from the peer-reviewed paper 10.3390/futurepharmacol2010006
This entry is offline, you can click here to edit this entry!
