Nanofluids obtained from halloysite and de-ionized water (DI) were prepared by using surfactants and changing pH for heat-transfer applications. The halloysite nanotubes (HNTs) nanofluids were studied for several volume fractions (0.5, 1.0, and 1.5 vol%) and temperatures (20, 30, 40, 50, and 60 °C).
- Halloysite, Nanofluids, Thermal conductivity
1. Introduction
Conventional heat-transfer fluids such as water, propylene glycol, ethylene glycol, and engine oil have been broadly utilized in many industrial applications. The heat-transfer enhancement of these fluids can reduce the material cost, energy, process time, and size, and increase the lifetime of the device [1][2].
In the heat-exchange systems, one of the problems is that the conventional heat-transfer fluids have low thermal conductivity. The thermal conductivity of these fluids can be enhanced by dispersing the solid particles. The study of the thermal conductivity of mixtures of solid particles and liquids was first developed in the 19th century when James Clerk Maxwell dispersed small particles into liquids. Further studies were conducted with millimeter, micro-sized particles. These particles enhance the properties of fluids. However, the major problem with these particles is that they settle rapidly in the fluids. Additionally, this causes a pressure drop and erosion of pipelines. The issues may be resolved by using nano-sized particles [3].
Nano-suspensions are the new class of nanotechnology-based heat-transfer fluid. First, aluminum oxide (Al2O3) ultrafine particles were dispersed into water by Masuda et al., and the thermal conductivity enhancement was 30% [4]. Then, the nanofluids were first introduced in 1995 by Choi et al. [5]. Since Choi introduced the concept of nanofluids, more researchers have started to search, develop and publish many articles about them. From 1993 to 2019 only, more than 11,000 articles were published and in 2019 the number of the published articles was 2005 [6].
Generally, nanofluids consist of two main parts: the nanoparticles and the base fluid. Nanofluids can be prepared from many different combinations, examples of the solid particles are metal- (metals: Al, Cu, Ag, etc.; metal oxides: Al2O3, CuO, TiO2, etc.; metal carbides: TiC), metalloid- (SiC, SiO2) and non-metal (carbon materials: graphite, diamond, graphene, etc.) based nanomaterials. Examples for the base fluids are water, ethylene glycol, ethanol, oil, and other conventional fluids. Nanoparticles are used to enhance the useful properties of liquids, modify their rheological behavior [6]. In the nanofluids, the nanoparticles have a complicated movement with coagulation, thermophoresis effect and Brownian motion. These factors depend on the concentration, temperature, size, shape, and type of nanoparticles, and so on. In the literature, it was shown that these factors play an important role in increasing the thermal conductivity and viscosity [7][8][9][10].
Nonetheless, the results from various research groups were different for the same materials. This can be explained by preparation techniques and the agglomeration state in nanofluids. Buongiorno et al. [3] performed benchmark research to compare the results of thermal conductivity obtained by different investigators. The same samples were measured in different locations, and with different methods, then the results and measurement error could be evaluated.
There is a lack of agreement between theory and experimental results. Some heat-transfer mechanisms have been proposed, such as liquid-layering, aggregation, particle motion, etc. [1]. In the aggregation mechanism, thermal conductivity occurs along with large particles or aggregates. This means that the size and shape of particles and clusters play an important role in thermal conductivity enhancement [11][12][13]. Because it was found that materials with chain-like structures, nanofibers or nanotubes have the highest thermal conductivity, much research has been performed on applications of CNTs nanofluids [14][15][16][17], titanium dioxide nanotube [18], titanate nanotube [19], halloysite nanotube nanofluids [20], etc. Venerus et al. [21] implemented the benchmark research for the comparison of viscosity values of the same samples from different research groups.
The use of nanoparticles improves the thermal conductivity of fluids and increases their viscosity, which causes an increase in pump energy. This limits the industrial application of nanofluids in heat-transfer systems. Like thermal conductivity, the viscosity of nanofluids also depends on the size and shape of particles and clusters. Therefore, the combined investigation of viscosity and thermal conductivity is essential. Many studies, including on both of these issues, have been performed [4][22][23][24].
Halloysite, belonging to the kaolin group, is a low-cost nanotubular clay with the chemical formula Al2Si2O5(OH)4·nH2O, where n = 0–2 [25]. The length of the halloysite is from 0.02 to 30 µm, the inner diameter is from 10 to 100 nm, and the outer diameter is approximately 30 to 190 nm [26][27]. The inner surface includes Al–OH groups, while the outer surface comprises inert Si–O–Si groups. Therefore, the reactivities of the outer and inner surfaces are different [28]. Because of these properties, there are many different applications of halloysite, such as solvent-free nanofluids [29][30], nanoreactors [31], drug delivery [32], energy storage devices, etc. [33]. However, the ability to apply halloysite as nanomaterials to prepare water-based nanofluids has rarely been investigated. Alberola et al. prepared the halloysite nanofluid and improved its stability by setting pH = 12. The studied temperature was from 40 to 80 °C. The thermal conductivity enhancement was 8% at 5% volume concentration and T = 80 °C, while the viscosity increased with halloysite content [20].
Usage of high pH for stabilization limits the applications of halloysite-based nanofluids. In this research, the halloysite-based nanofluid was investigated by dispersing halloysite into the water, and the stability was improved by different surfactants. As far as authors know, there are no studies on stabilizing halloysite nanofluids with surfactants and investigations on heat-transfer applications. In addition, the size of halloysite used in this research is smaller than in previous research. The halloysite nanotube (HNT) was first analyzed by scanning electron microscopy, Fourier-transform infrared spectroscopy, X-ray powder diffraction, energy-dispersive X-ray analysis, and thermogravimetric analysis. The concentrations of nanofluids were prepared from 0.5 to 1.5 vol%. The thermal conductivity and dynamic viscosity of these nanofluids were measured. The temperatures during the experiments are from 20 to 60 °C. In order to evaluate the measurement results, the nanofluids were prepared with pH = 12 and the same concentrations.
2. Rheological Properties of Halloysite Nanofluid
The rheology and viscosity of the nanofluids are important parameters determining the heat transfer. The viscosity of the base fluid and HNT nanofluids at different shear rates is measured for three volume concentrations of 0.5, 1.0, and 1.5 at five temperatures: 20, 30, 40, 50, and 60 °C. Figure 1 shows the shear rate–shear stress diagram of 0.5 vol% HNT nanofluids with surfactant at different temperatures. Shear stress of HNT nanofluids falls with increasing temperature and rises with increasing concentration of nanofluids. The increase in temperature causes the Brownian movement and thermal motion of molecules to be higher, thus the viscosity of nanofluids decreases [34][35]. The shear rate of HNT nanofluid is almost linearly dependent on the shear rate. We conclude that the nanofluids are Newtonian.
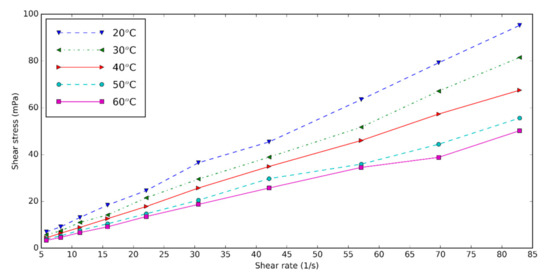
Figure 1. Shear stress–shear rates diagram of nanofluids for concentration of 0.5%.
Figure 2 shows the viscosity increment of prepared HNT nanofluids at different temperatures. Relative viscosity is obtained by dividing the viscosity of nanofluids by that of the base fluids. It can be seen that the viscosity is higher with nanoparticle content due to the clusters formed from nanoparticles [6]. Temperature plays an essential role in the relative viscosity. This means that temperature decreases the viscosity of base fluids more than that of the nanofluids. Compared to the HNT nanofluids at pH = 12, the relative viscosity of nanofluids containing the surfactant doesn’t have a significant difference. With surfactant, the HNT nanofluids have the lowest relative viscosity of 1.09 for 0.5% volume concentration and the highest relative viscosity of 1.31 for 1.5 vol%. The viscosity of nanofluids increased from 9% to 31% compared to the base fluid containing the surfactants.
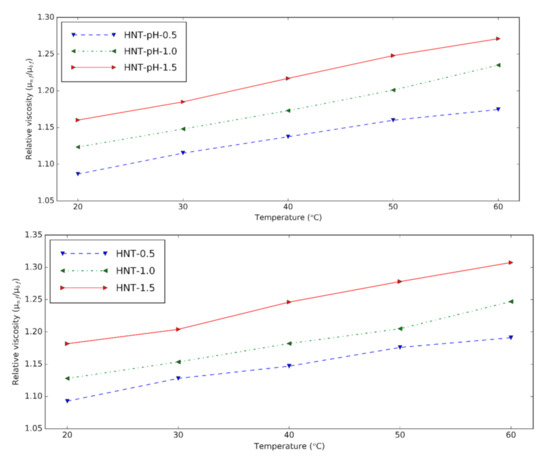
Figure 2. Relative viscosity of HNT nanofluids at different temperatures (upper: changing pH, lower: using surfactant).
3. Thermal Conductivity of Halloysite Nanofluid
The thermal conductivity of HNT nanofluids at different temperatures is presented in Figure 3. The device is reliable within 0.6% error when the calibration measurement is verified for distilled water. The nanofluids show greater thermal conductivity than the base fluid at experimental temperatures. HNT nanofluids with surfactant give 4.48%, 6.03% and 7.93% thermal conductivity increment at 0.5 vol%, 1.0 vol%, and 1.5 vol% in comparison with the base fluid at 20 °C, respectively. By increasing the temperature, the thermal conductivity of nanofluids increases due to the augmentation in the Brownian motion of the solid.
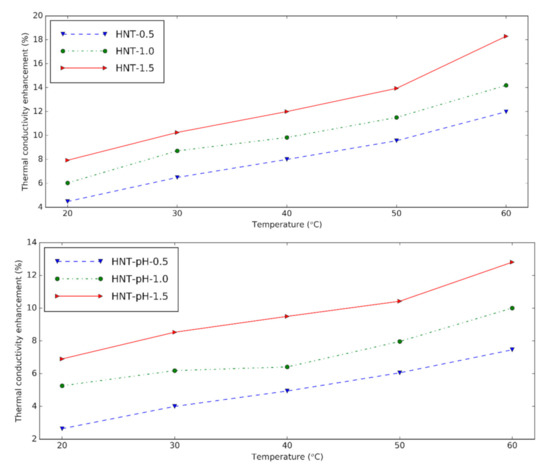
Figure 3. Thermal conductivity and enhancement of thermal conductivity of halloysite nanofluids at different temperatures (upper: changing pH, lower: using surfactant).
It can be seen that when the nanoparticle content in nanofluids increases, the thermal conductivity also increases because of the higher number of nanoparticles presented in the nanofluid. The thermal conductivity enhancement of nanofluids containing surfactant is slightly higher than nanofluids with pH = 12. It is concluded that like changing pH, surfactant supports using HNT in the preparation of nanofluids. Compared to the results from Alberola et al. [20], the thermal conductivity of the nanofluids in this study is greater. This may be due to the smaller halloysite used in this research.
4. Conclusions
With surfactants, the HNT nanofluids have the highest thermal conductivity increment of 18.30% for 1.5 vol% concentration in comparison with the base fluid. The thermal conductivity enhancement of nanofluids containing surfactant is slightly higher than nanofluids with pH = 12. From the rheological measurements, it is shown that the nanofluids were Newtonian. The viscosity enhancements of the nanofluid were 11% and 12.8% at 30 °C for 0.5% volume concentration with surfactants and pH = 12, respectively. Instead of changing pH, the surfactants give good results for the preparation of the nanofluid. Novel equations of viscosity and thermal conductivity for these nanofluids were proposed.
This entry is adapted from the peer-reviewed paper 10.3390/nano10091834
References
- Fan, J.; Wang, L. Review of Heat Conduction in Nanofluids. J. Heat Transf. 2011, 133.
- Godson, L.; Raja, B.; Lal, D.M.; Wongwises, S. Enhancement of heat transfer using nanofluids—An overview. Renew. Sustain. Energy Rev. 2010, 14, 629–641.
- Buongiorno, J.; Venerus, D.C.; Prabhat, N.; McKrell, T.; Townsend, J.; Christianson, R.; Tolmachev, Y.V.; Keblinski, P.; Hu, L.-W.; Alvarado, J.L.; et al. A benchmark study on the thermal conductivity of nanofluids. J. Appl. Phys. 2009, 106, 094312.
- Masuda, N.H.H.; Ebata, A.; Teramae, K. Alteration of thermal conductivity and viscosity of Liquid by dispersing ultra fine particles. Netsu Bussei 1993, 7, 227–233.
- Xuan, Y.; Li, Q. Investigation on Convective Heat Transfer and Flow Features of Nanofluids. J. Heat Transf. 2003, 125, 151–155.
- Le Ba, T.; Mahian, O.; Wongwises, S.; Szilágyi, I.M. Review on the recent progress in the preparation and stability of graphene-based nanofluids. J. Therm. Anal. Calorim. 2020, 1–28.
- Jang, S.P.; Choi, S.U.S. Role of Brownian motion in the enhanced thermal conductivity of nanofluids. Appl. Phys. Lett. 2004, 84, 4316.
- Shukla, R.K.; Dhir, V.K. Effect of Brownian Motion on Thermal Conductivity of Nanofluids. J. Heat Transf. 2008, 130, 042406.
- Lin, Y.; Jiang, Y. Effects of Brownian motion and thermophoresis on nanofluids in a rotating circular groove: A numerical simulation. Int. J. Heat Mass Transf. 2018, 123, 569–582.
- Koo, J.; Kleinstreuer, C. Impact analysis of nanoparticle motion mechanisms on the thermal conductivity of nanofluids. Int. Commun. Heat Mass Transf. 2005, 32, 1111–1118.
- Shima, P.D.; Philip, J.; Raj, B. Influence of aggregation on thermal conductivity in stable and unstable nanofluids. Appl. Phys. Lett. 2010, 97, 153113.
- Gao, J.; Zheng, R.T.; Ohtani, H.; Zhu, D.S.; Chen, G. Experimental Investigation of Heat Conduction Mechanisms in Nanofluids. Clue on Clustering. Nano Lett. 2009, 9, 4128–4132.
- Wu, C.; Cho, T.-J.; Xu, J.; Lee, D.; Yang, B.; Zachariah, M.R. Effect of nanoparticle clustering on the effective thermal conductivity of concentrated silica colloids. Phys. Rev. E 2010, 81, 011406.
- Askari, S.; Lotfi, R.; Seifkordi, A.; Rashidi, A.; Koolivand, H. A novel approach for energy and water conservation in wet cooling towers by using MWNTs and nanoporous graphene nanofluids. Energy Convers. Manag. 2016, 109, 10–18.
- Verma, S.K.; Tiwari, A.K.; Chauhan, D.S. Experimental evaluation of flat plate solar collector using nanofluids. Energy Convers. Manag. 2017, 134, 103–115.
- Haque, A.K.M.M.; Kim, T.; Oh, G.S.; Kim, J.; Noh, J.; Choi, B.; Chung, H.; Jeong, H.; Huh, S.; Mahmudul, H.A.K.M.; et al. Synthesis of Graphene/Multi-Walled Carbon Nanotube Composite and Its Nanofluid Preparation. Nanosci. Nanotechnol. Lett. 2016, 8, 316–323.
- Ding, Y.; Alias, H.; Wen, D.; Williams, R. Heat transfer of aqueous suspensions of carbon nanotubes (CNT nanofluids). Int. J. Heat Mass Transf. 2006, 49, 240–250.
- Shao, X.; Chen, Y.; Mo, S.; Cheng, Z.; Yin, T. Dispersion Stability of TiO2-H2O Nanofluids Containing Mixed Nanotubes and Nanosheets. Energy Procedia 2015, 75, 2049–2054.
- Chen, H.; Ding, Y.; Lapkin, A.; Fan, X. Rheological behaviour of ethylene glycol-titanate nanotube nanofluids. J. Nanopart. Res. 2009, 11, 1513–1520.
- Alberola-Borràs, J.-A.; Mondragon, R.; Julia, J.E.; Hernández, L.; Cabedo, L. Characterization of halloysite-water nanofluid for heat transfer applications. Appl. Clay Sci. 2014, 99, 54–61.
- Venerus, D.C.; Buongiorno, J.; Christianson, R.; Townsend, J.; Bang, I.C.; Chen, G.; Chung, S.J.; Chyu, M.; Chen, H.; Ding, Y.; et al. Viscosity measurements on colloidal dispersions (nanofluids) for heat transfer applications. Appl. Rheol. 2010, 20, 2.
- Hamid, K.A.; Azmi, W.; Nabil, M.; Mamat, R.; Sharma, K. Experimental investigation of thermal conductivity and dynamic viscosity on nanoparticle mixture ratios of TiO2-SiO2 nanofluids. Int. J. Heat Mass Transf. 2018, 116, 1143–1152.
- Turgut, A.; Tavman, I.; Chirtoc, M.; Karbstein, H.; Sauter, C.; Tavman, S. Thermal Conductivity and Viscosity Measurements of Water-Based TiO2 Nanofluids. Int. J. Thermophys. 2009, 30, 1213–1226.
- Nabil, M.; Azmi, W.; Hamid, K.A.; Mamat, R.; Hagos, F.Y.; Mohamad, M.N.F. An experimental study on the thermal conductivity and dynamic viscosity of TiO2 -SiO2 nanofluids in water: Ethylene glycol mixture. Int. Commun. Heat Mass Transf. 2017, 86, 181–189.
- Kluger, M.O.; Moon, V.; Kreiter, S.; Lowe, D.J.; Churchman, G.; Hepp, D.A.; Seibel, D.; Jorat, M.E.; Mörz, T. A new attraction-detachment model for explaining flow sliding in clay-rich tephras. Geology 2016, 45, 131–134.
- Yuan, P.; Tan, D.; Annabi-Bergaya, F. Properties and applications of halloysite nanotubes: Recent research advances and future prospects. Appl. Clay Sci. 2015, 112, 75–93.
- Yuan, P.; Southon, P.D.; Liu, Z.; Green, M.E.R.; Hook, J.M.; Antill, S.J.; Kepert, C.J. Functionalization of Halloysite Clay Nanotubes by Grafting with γ-Aminopropyltriethoxysilane. J. Phys. Chem. C 2008, 112, 15742–15751.
- Tan, D.; Yuan, P.; Liu, D.; Du, P. Surface Modifications of Halloysite; Elsevier: Amsterdam, The Netherlands, 2016.
- Yang, S.; Li, S.; Yin, X.; Wang, L.; Chen, D.; Zhou, Y.; Wang, H. Preparation and characterization of non-solvent halloysite nanotubes nanofluids. Appl. Clay Sci. 2016, 126, 215–222.
- Du, P.; Liu, D.; Yuan, P.; Deng, L.; Wang, S.; Zhou, J.; Zhong, X. Controlling the macroscopic liquid-like behaviour of halloysite-based solvent-free nanofluids via a facile core pretreatment. Appl. Clay Sci. 2018, 156, 126–133.
- Shchukin, D.; Sukhorukov, G.; Price, R.; Lvov, Y. Halloysite Nanotubes as Biomimetic Nanoreactors. Small 2005, 1, 510–513.
- Li, X.; Yang, Q.; Ouyang, J.; Yang, H.; Chang, S. Chitosan modified halloysite nanotubes as emerging porous microspheres for drug carrier. Appl. Clay Sci. 2016, 126, 306–312.
- Deen, I.; Pang, X.; Zhitomirsky, I. Electrophoretic deposition of composite chitosan–halloysite nanotube–hydroxyapatite films. Colloids Surfaces A Physicochem. Eng. Asp. 2012, 410, 38–44.
- Afrand, M.; Toghraie, D.; Ruhani, B. Effects of temperature and nanoparticles concentration on rheological behavior of Fe3O4–Ag/EG hybrid nanofluid: An experimental study. Exp. Therm. Fluid Sci. 2016, 77, 38–44.
- Nguyen, C.; Desgranges, F.; Roy, G.; Galanis, N.; Mare, T.; Boucher, S.; Mintsa, H.A. Temperature and particle-size dependent viscosity data for water-based nanofluids–Hysteresis phenomenon. Int. J. Heat Fluid Flow. 2007, 28, 1492–1506.
