Functional osseoconductive coatings based on hydroxylapatite (HAp) and applied preferentially by atmospheric plasma spraying to medical implant surfaces are a mainstay of modern implantology. During contact with the hot plasma jet, HAp particles melt incongruently and undergo complex dehydration and decomposition reactions that alter their phase composition and crystallographic symmetry, and thus, the physical and biological properties of the coatings. Surface analytical methods such as laser-Raman and nuclear magnetic resonance (NMR) spectroscopies are useful tools to assess the structural changes of HAp imposed by heat treatment during their flight along the hot plasma jet.
- hydroxylapatite
- oxyhydroxylapatite
- oxyapatite
- tricalcium phosphate
- tetracalcium phosphate
- Raman spectroscopy
- MAS-CP NMR spectroscopy
- 2D-HETCOR NMR spectroscopy
1. Introduction
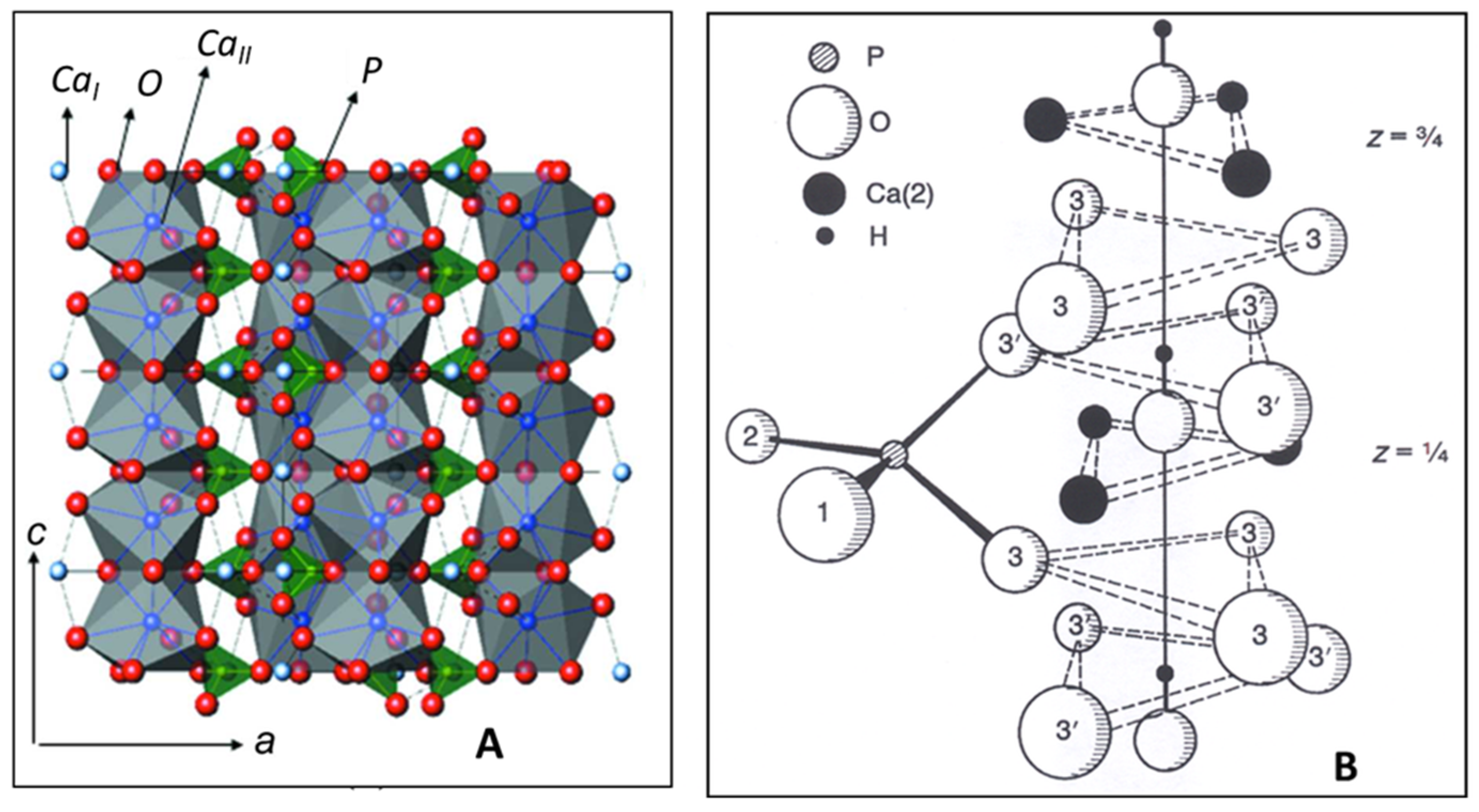
2. Structure of Hydroxylapatite
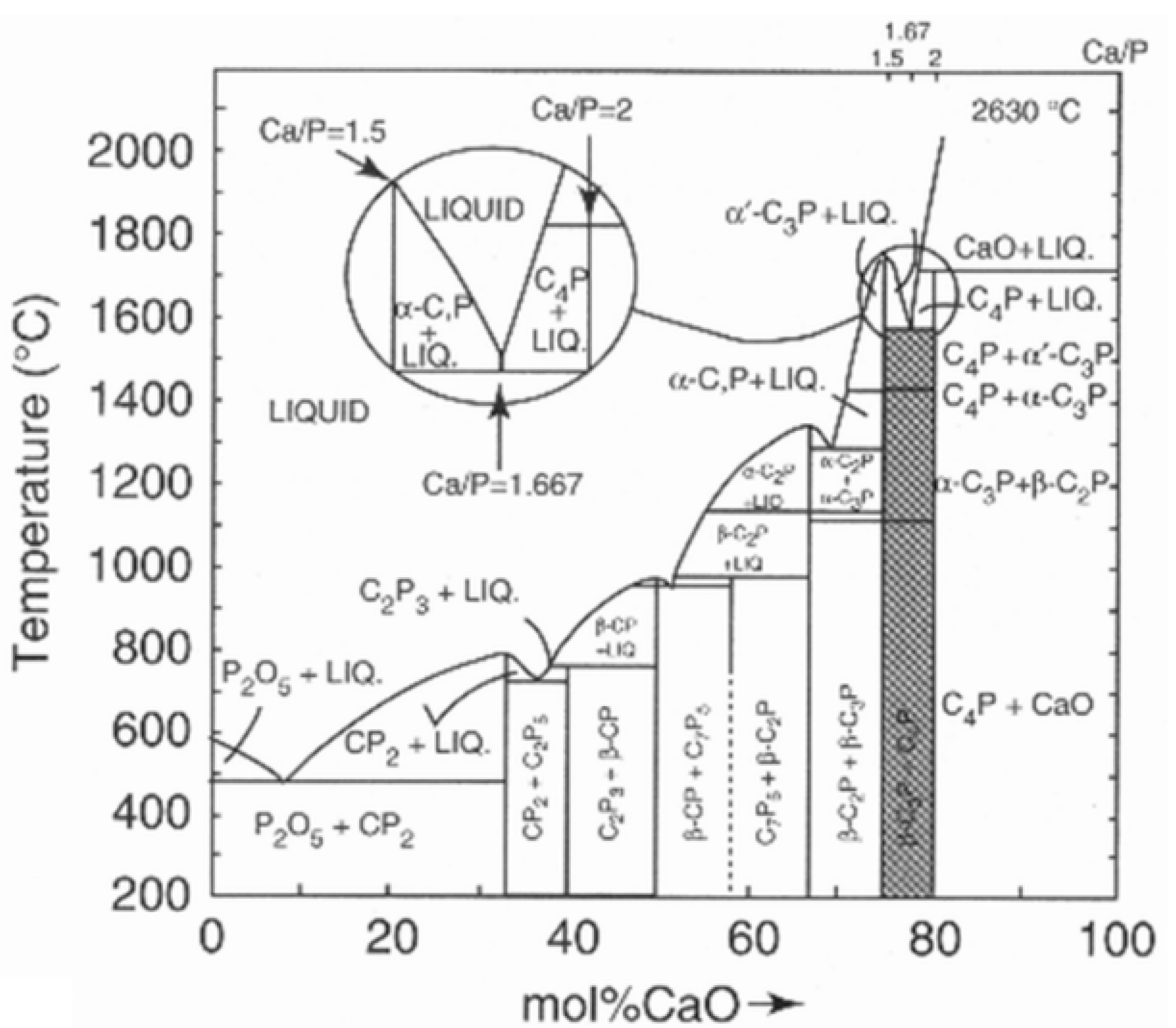
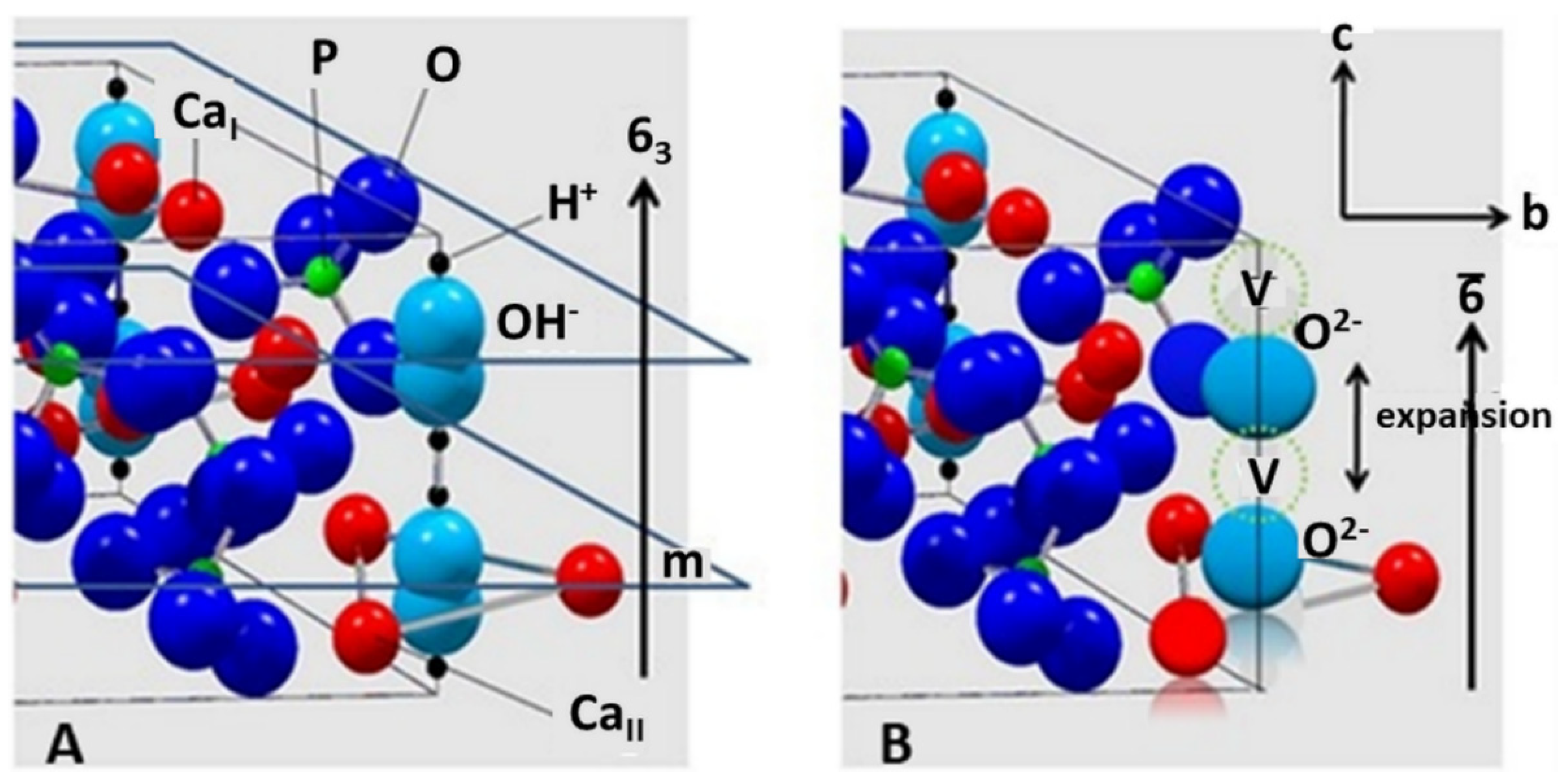
3. Thermal Alteration during Plasma Spraying
| Phase | S.G. | a0 (nm) | b0 (nm) | c0 (nm) | α (o) | ß (o) | γ (o) | Reference |
|---|---|---|---|---|---|---|---|---|
| HAp | P63/m | 0.9432 | 0.9432 | 0.6881 | 120 | [24] | ||
| HAp | P63/m | 0.9418 | 0.9418 | 0.68827 | 120 | [25] | ||
| c-empty * | P63/m | 0.910 | 0.910 | 0.682 | 120.1 | [26] | ||
| m-HAp | P21/b | 0.9421 | 2a0 | 0.6881 | 120 | [27] | ||
| OHAp | P1¯ | 0.9400 | 0.9397 | 0.6899 | 90.063 | 89.748 | 119.997 | [18] |
| OAp | P6¯ | 0.9432 | 0.9432 | 0.6881 | 120 | [28] | ||
| OAp | P6¯ | n.d. | n.d. | 0.6902 | n.d. | [29] | ||
| OAp | P6¯ | n.d. | n.d. | 0.6931 | n.d. | [27] | ||
| OAp * | P6¯ | 0.906 | 0.906 | 0.673 | 90.03 | 90.00 | 119.9 | [26] |
| TTCP | P21 | 0.7023 | 1.1986 | 0.9473 | 90.901 | [30] | ||
| α-TCP | P21/a | 1.2873 | 2.7280 | 1.5213 | 126.208 | [31] | ||
| ß-TCP | R3c | 1.0435 | 1.0435 | 3.7403 | 120 | [32] |
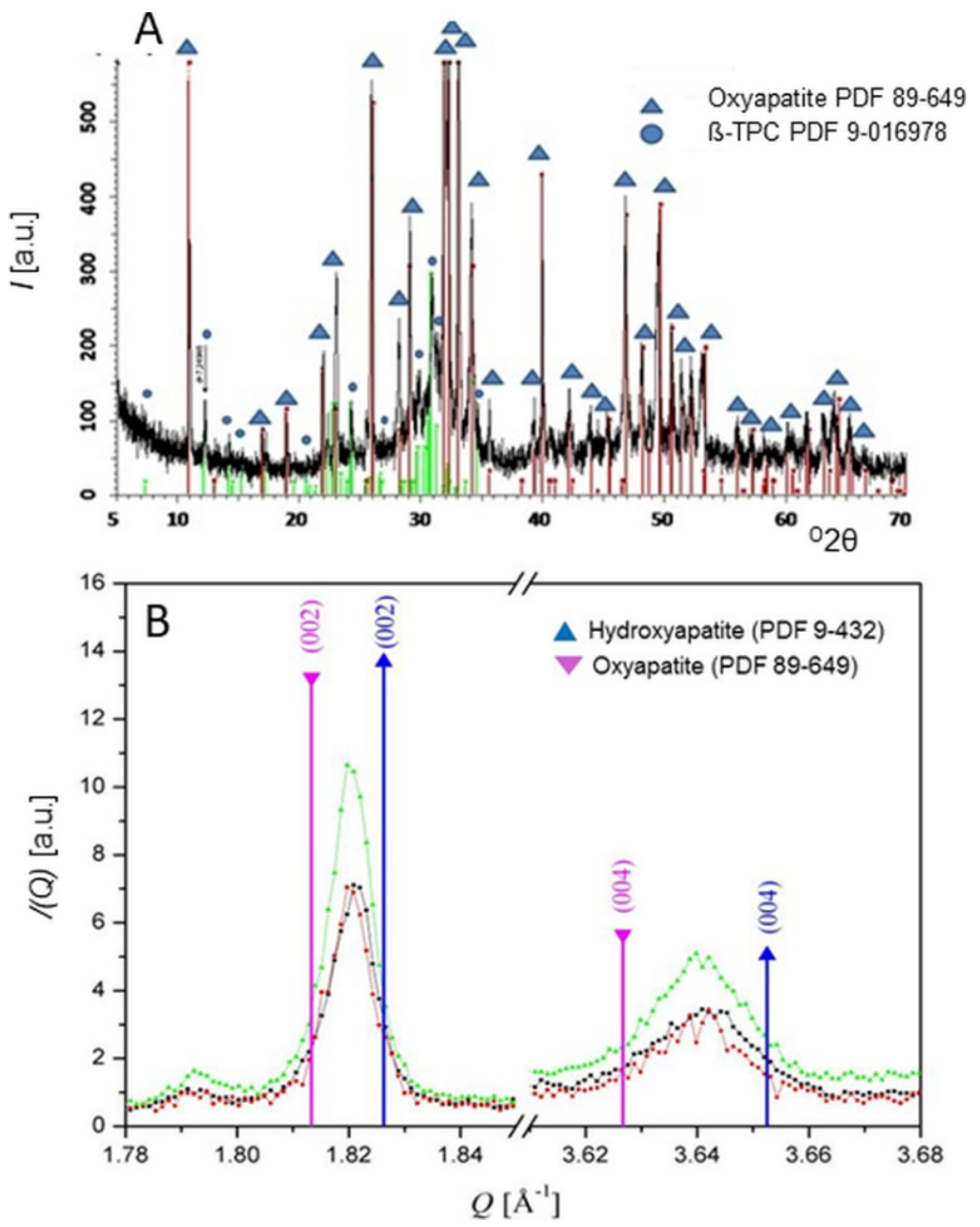
This entry is adapted from the peer-reviewed paper 10.3390/coatings11080987
References
- Silva, C.C.; Sombra, S. Raman spectroscopy measurements of hydroxyapatite obtained by mechanical alloying. J. Phys. Chem. Sol. 2004, 65, 1031–1033.
- Ulian, G.; Valdrè, G.; Como, M.; Ugliengo, P. The vibrational features of hydroxylapatite and type A carbonated apatite: A first principle contribution. Am. Mineral. 2013, 98, 752–759.
- Nosenko, V.V.; Yaremko, A.M.; Dzhagan, V.M.; Vorona, I.P.; Romanyuk, Y.A.; Zatovsky, I.V. Nature of some features in Raman spectra of hydroxyapatite-containing materials. J. Raman Spectrosc. 2016, 47, 726–730.
- Antonakos, A.; Liarokapis, E.; Kyriacou, A.; Leventouri, T. Raman and IR studies of the effect of Fe substitution in hydroxyapatite and deuterated hydroxyapatite. Am. Mineral. 2017, 102, 85–91.
- Mohonta, S.K.; Maria, K.H.; Rahman, S.; Das, H.; Hoque, S.M. Synthesis of hydroxyapatite nanoparticles and role of its site in hydroxyapatite/chitosan-gelatin biocomposite for bone grafting. Int. Nano Lett. 2021.
- Stammeier, J.A.; Purgstaller, B.; Hippler, D.; Mavromatis, V.; Dietzel, M. In-Situ Raman spectroscopy of amorphous calcium phosphate to crystalline hydroxyapatite transformation. MethodsX 2018, 5, 1241–1250.
- Rothwell, W.P.; Waugh, J.S.; Yesinowski, J.P. High-resolution variable-temperature 31P NMR of solid calcium phosphates. J. Am. Chem. Soc. 1980, 102, 2637–2643.
- Yesinowski, J.P.; Wolfgang, R.A.; Mobley, M.J. New NMR methods for the study of hydroxyapatite surfaces. In Adsorption on and Surface Chemistry of Hydroxyapatite; Misra, D.N., Ed.; Springer: Boston, MA, USA, 1984; pp. 151–175.
- Mason, H.E.; Kozlowski, A.; Phillips, B.L. Solid-state NMR study of the role of H and Na in AB-type carbonate hydroxylapatite. Chem. Mater. 2007, 20, 294–302.
- Chappell, H.; Duer, M.; Groom, N.; Pickard, C.; Bristowe, P. Probing the surface structure of hydroxyapatite using NMR spectroscopy and first principles calculations. Phys. Chem. Chem. Phys. 2008, 10, 600–606.
- Jäger, C.; Welzel, T.; Meyer-Zaika, W.; Epple, M. A solid-state NMR investigation of the structure of nanocrystalline hydroxyapatite. Magn. Reson. Chem. 2006, 44, 573–580.
- Lu, H.B.; Campbell, C.T.; Graham, D.J.; Ratner, B.D. Surface characterization of hydroxyapatite and related calcium phosphates by XPS and TOF-SIMS. Anal. Chem. 2000, 72, 2886–2894.
- Young, R.A.; Elliott, J.C. Atomic-scale bases for several properties of apatites. Arch. Oral Biol. 1966, 11, 699–707.
- Veselinovic, L.; Karanovic, L.; Stojanovic, Z.; Bracko, I.; Markovic SIgnatovic, N.; Uskokovic, D. Crystal structure of cobalt-substituted calcium hydroxyapatite nanopowders prepared by hydrothermal processing. J. Appl. Cryst. 2010, 43, 320–327.
- Elliott, J.C.; Wilson, R.M.; Dowker, S.E.P. Apatite structures. Adv. X-ray Anal. 2002, 45, 172–181.
- Kreidler, E.R.; Hummel, F.A. Phase relations in the system SrO-P2O5 and the influence of water vapor on the formation of Sr4P2O9. Inorg. Chem. 1967, 6, 884–891.
- Heimann, R.B. Plasma Spray Coating. Principles and Applications, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2008; p. 34.
- Alberius-Henning, P.; Adolfson, E.; Grins, J.; Fitch, A. Triclinic oxy-hydroxyapatite. J. Mater. Sci. 2001, 36, 663–668.
- Liao, C.J.; Lin, F.H.; Chen, K.S.; Sun, J.S. Thermal decomposition and reconstruction of hydroxyapatite in air atmosphere. Biomaterials 1999, 20, 1807–1813.
- Trombe, J.C.; Montel, G. Sur la preparation de l’oxyapatite phosphocalcique. Comptes Rendus Séances l’Académie Sci. (Paris) Série C 1971, 273, 452–465.
- Hartmann, P.; Jäger, C.; Barth, S.; Vogel, J.; Meyer, K. Solid state NMR, X-ray diffraction, and infrared characterization of local structure in heat-treated oxyhydroxylapatite microcrystals: An analogy of the thermal deposition of hydroxyapatite during plasma-spray procedure. J. Solid State Chem. 2001, 160, 460–468.
- Heimann, R.B.; Tran, H.V.; Hartmann, P. Laser-Raman and Nuclear Magnetic Resonance (NMR) studies on plasma-sprayed hydroxyapatite coatings: Influence of bioinert bond coats on phase composition and resorption kinetics in simulated body fluid. Entwickl. Fert. Prüfung Eig. Anwend. Tech. Werkst. 2003, 34, 1163–1169.
- Kijima, T.; Tsutsumi, M. Preparation and thermal properties of dense polycrystalline oxyhydroxyapatite. J. Am. Ceram. Soc. 1979, 62, 455–460.
- Posner, A.S.; Perloff, A.; Diorio, A.F. Refinement of the hydroxyapatite structure. Acta Cryst. 1958, 11, 308–309.
- Tas, A.C. X-ray diffraction data for flux-grown calcium hydroxyapatite whiskers. Powder Diffr. 2001, 16, 102–106.
- Calderin, L.; Stott, M.J.; Rubio, A. Electronic and crystallographic structure of apatite. Phys. Rev. B 2003, 67, 134106–134112.
- Elliott, J.C.; Mackie, P.E.; Young, R.A. Monoclinic hydroxylapatite. Science 1973, 180, 1055–1057.
- Alberius-Henning, P.; Landa-Canovas, A.; Larsson, A.K.; Lidin, S. The structure of oxyapatite solved by HREM. Acta Crystallogr. B 1999, 55, 170–176.
- Heimann, R.B. Characterisation of as-sprayed and incubated hydroxyapatite coatings with high resolution techniques. Mater. Werkst. 2009, 40, 23–30.
- Dickens, B.; Brown, W.E.; Kruger, G.J.; Stewart, J.M. Ca4(PO4)2O, tetracalcium diphosphate monoxide. Crystal structure and relationships to Ca5(PO4)3OH and K3Na(SO4)2. Acta Cryst. 1973, 29, 2046–2056.
- Yashima, M.; Kawaike, Y.; Tanaka, M. Determination of precise unit cell parameters of the α-tricalcium phosphate Ca3(PO4)2 through high-resolution synchrotron powder diffraction. J. Am. Ceram. Soc. 2007, 90, 272–274.
- Yashima, M.; Sakai, A.; Kamiyama, T.; Hoshikawa, A. Crystal structure analysis of ß-tricalcium phosphate Ca3(PO4)2 by neutron diffraction. J. Solid State Chem. 2003, 175, 272–277.
- Hendricks, S.B.; Hill, W.A.; Jakobs, K.D.; Jefferson, M.E. Structural characteristics of apatite-like substances and composition of phosphate rock and bone as determined from microscopical and X-ray examinations. Ind. Eng. Chem. 1931, 23, 1413–1418.
- Voelcker, J.A. Die chemische Zusammensetzung des Apatits nach eigenen vollständigen Analysen. Ber. Dtsch. Chem. Ges. 1883, 16, 2460–2464.
- Rogers, A.F. A new locality for voelckerite and the validity of voelckerite as a mineral species. Mineral. Mag. J. Mineral. Soc. 1914, 17, 155–162.
- Bredig, M.A.; Franck, H.H.; Füldner, H. Beiträge zur Kenntnis der Kalk-Phosphorsäure-Verbindungen II. Z. Für Elektrochem. Angew. Phys. Chem. 1933, 39, 959–969.
- De Leeuw, N.; Bowe, J.R.; Rabone, J.A.L. A computational investigation of stoichiometric and calcium-deficient oxy- and hydroxyapatite. Faraday Disc. 2007, 134, 195–214.
- McConnell, D.; Hey, M.H. The oxyapatite (voelckerite) problem. Min. Mag. 1969, 37, 301–303.
- Trombe, J.C.; Montel, G. Some features of the incorporation of oxygen in different oxidation states in the apatite lattice. I. On the existence of calcium and strontium oxyapatite. J. Inorg. Nucl. Chem. 1978, 40, 15–21.
- Gross, K.A.; Berndt, C.C.; Stephens, P.; Dinnebier, R. Oxyapatite in hydroxyapatite coatings. J. Mater. Sci. 1998, 33, 3985–3991.
- Gross, K.A.; Ben-Nissan, B.; Walsh, W.R.; Swarts, E. Analysis of retrieved hydroxyapatite coated orthopaedic implants. In Thermal Spray. Meeting the Challenges of the 21st Century; Coddet, C., Ed.; ASM International: Almere, The Netherlands, 1998; pp. 1133–1138.
- Gross, K.A.; Berndt, C.C. Thermal processing of hydroxyapatite for coating production. J. Biomed. Mater. Res. 1998, 39, 580–587.
- Trombe, J.C. Contribution á l’étude de la decomposition et de la réactivité de certaines apatites hydroxylées et carbonates. Ann Chim. (Paris) 1973, 8, 335–347.
- Montel, G.; Bonel, G.; Trombe, J.C.; Heughebaert, J.C.; Rey, C. Progress dans le domaine de la chimie des composes phosphores solides a structure d’apatite. Pure Appl. Chem. 1980, 52, 973–987.
- Keller, L.; Dollase, W.A. X-ray determination of crystalline hydroxyapatite to amorphous calcium phosphate ratio in plasma sprayed coatings. J. Biomed. Mater. Res. 2000, 49, 244–249.
