Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Ophthalmology
Many strategies were designed in terms of the therapeutic modality of ocular diseases. The advent of nanotechnology-based therapeutic systems has acquainted the novel facet toward the optimized nanosize particle, which enables minimizing irritation, addressing the poor bioavailability, and improving ocular biocompatibility of therapeutics.
- ocular therapeutic system
- drug delivery
- hydrogel
- nanomicelles
- nanoparticles
1. Introduction
Many strategies were designed in terms of the therapeutic modality of ocular diseases. The advent of nanotechnology-based therapeutic systems has acquainted the novel facet toward the optimized nanosize particle, which enables minimizing irritation, addressing the poor bioavailability, and improving ocular biocompatibility of therapeutics. The various nanodrug delivery carrier systems employed in the ocular therapeutic system are presented in Figure 1. The nanocarriers enlisted below have shown promised results for enriching the ocular availability of therapeutics.
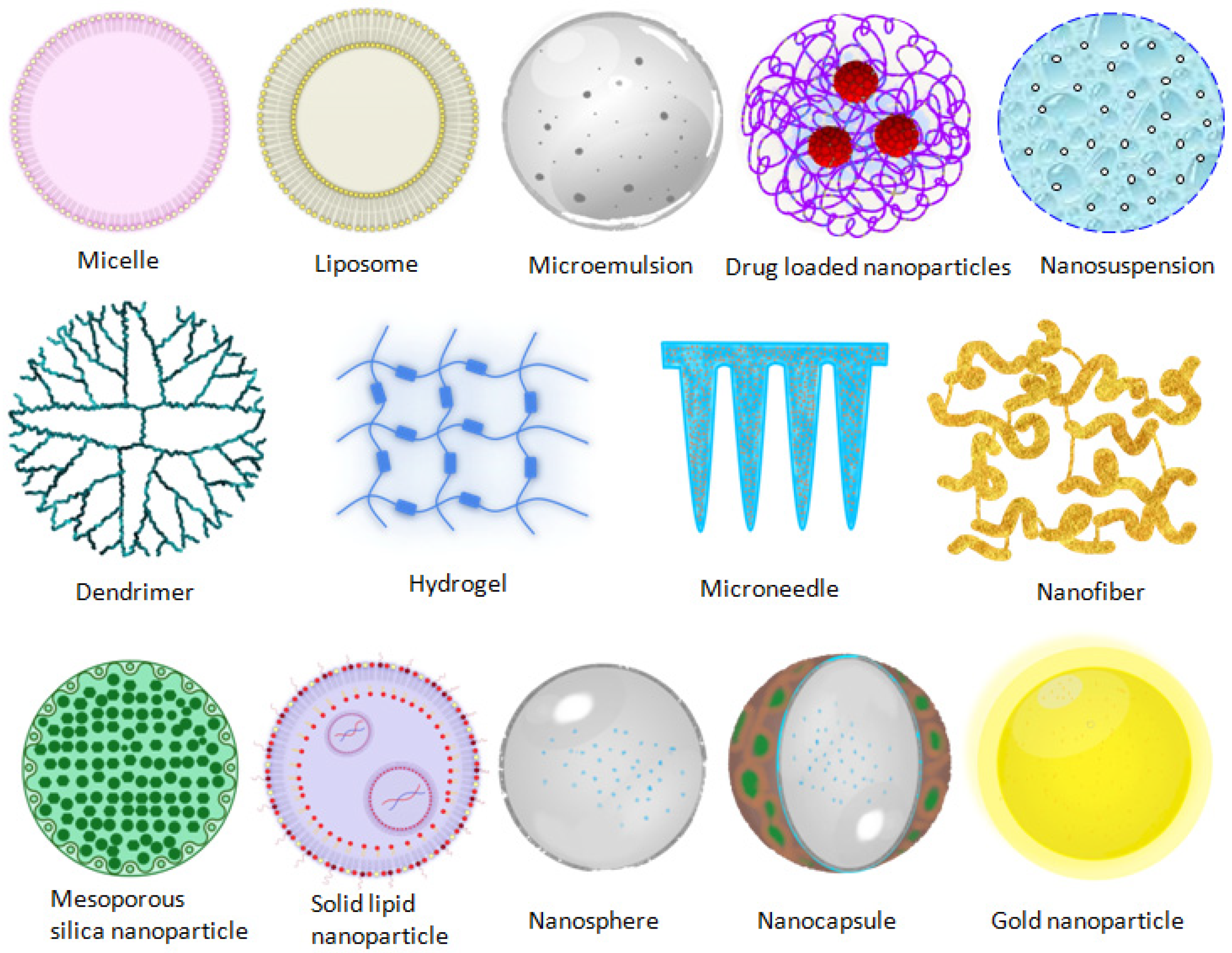
Figure 1. Nanocarriers employed in ocular therapeutic systems.
2. Microemulsion
MEs comprise of oil, surfactant, and cosurfactant with medicaments. In general, the thermodynamic stability of oil in water emulsion (o/w) is a consequence of interfacial film around water droplets [1]. ME as a drug delivery vehicle for eye preparation has been widely explored to overcome the various barriers in ocular drug delivery. To improve the significant concentration of drug in the posterior chamber of the eye, an overwhelming study led by Nayak and Misra investigated the PEGylated ME delivery into the posterior segment of the eye (Figure 2). They developed TA-loaded ME, post evaluation in vitro for solubility, emulsion capability, and they construed a pseudoternary phase diagram. The optimum formulation comprised a ratio of oil (Capmul MCM C8): surfactant (AccononMC8-2): cosurfactant (Transcutol): water of 5:35.5:4.5:55. The emulsion was PEGylated using 1, 2-distearoylphosphatylethanolamine-polyethyleneglycol 2000 (DSPE-PEG 2000). Moreover, the PEGylated drug-loaded ME was characterized and investigated for topical application. The prepared PEGylated ME was observed to be stable, homogenous, and nonirritant to the eye, after animal study, and had the potential to achieve the target to the posterior segment of the eye after topical administration [2].
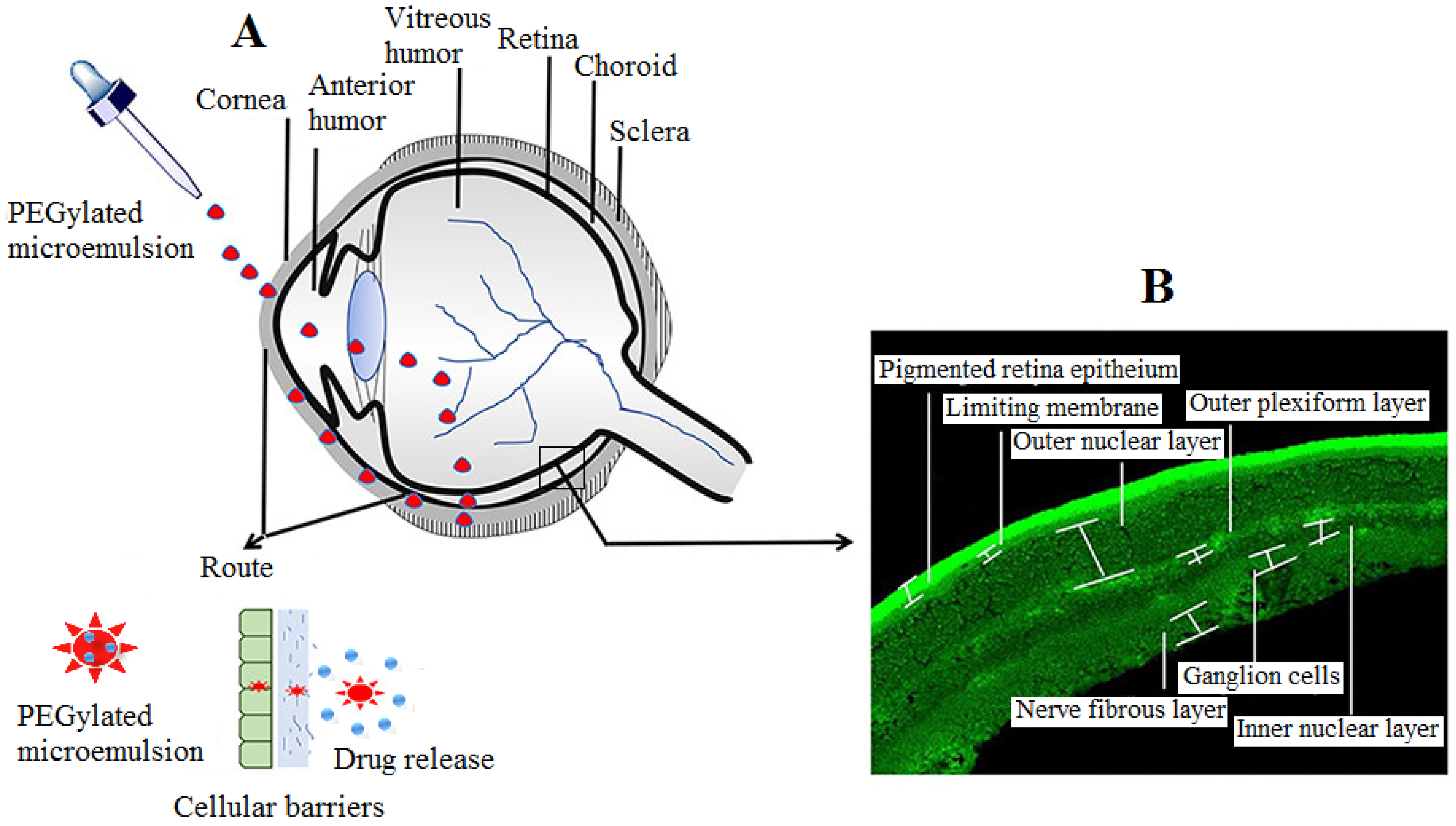
Figure 2. Diagram showing the route of PEGylated ME entry into the posterior segment of eye (A) and different parts of retina of eye (B). To overcome the cellular barriers, topical PEGylated ME may cross the membrane barriers viz. cornea, conjunctiva, and sclera, thereby preventing opsonization and improving circulation in lachrymal fluid and vitreous humor. Modified from ref. [2]; permission granted from ACS publishers (https://pubs.acs.org/doi/10.1021/acsomega.9b04244.
The findings shown in Figure 2 illustrate that aseptically developed ME was free of microbial contamination and did not corroborate the microbial growth. In the isotonicity test shown in Figure 2II, the architecture of RBC is maintained in both normal ME and PEGylated ME preparation, which confirmed that both formulations were isotonic to ocular fluid, while they were ruptured in hypertonic (B) and swollen in hypotonic solution (C). The blood and tear fluids possess the same osmolarity, and thus, RBC was utilized for the isotonicity test. Figure 2III shows the nuclei of cornea incubated with normal ME and PEGylated ME were safe, with no risk of side effects using them. Figure 2IV shows the corneal hydration and staining test, which confirmed the nonirritant nature of formulations [2].
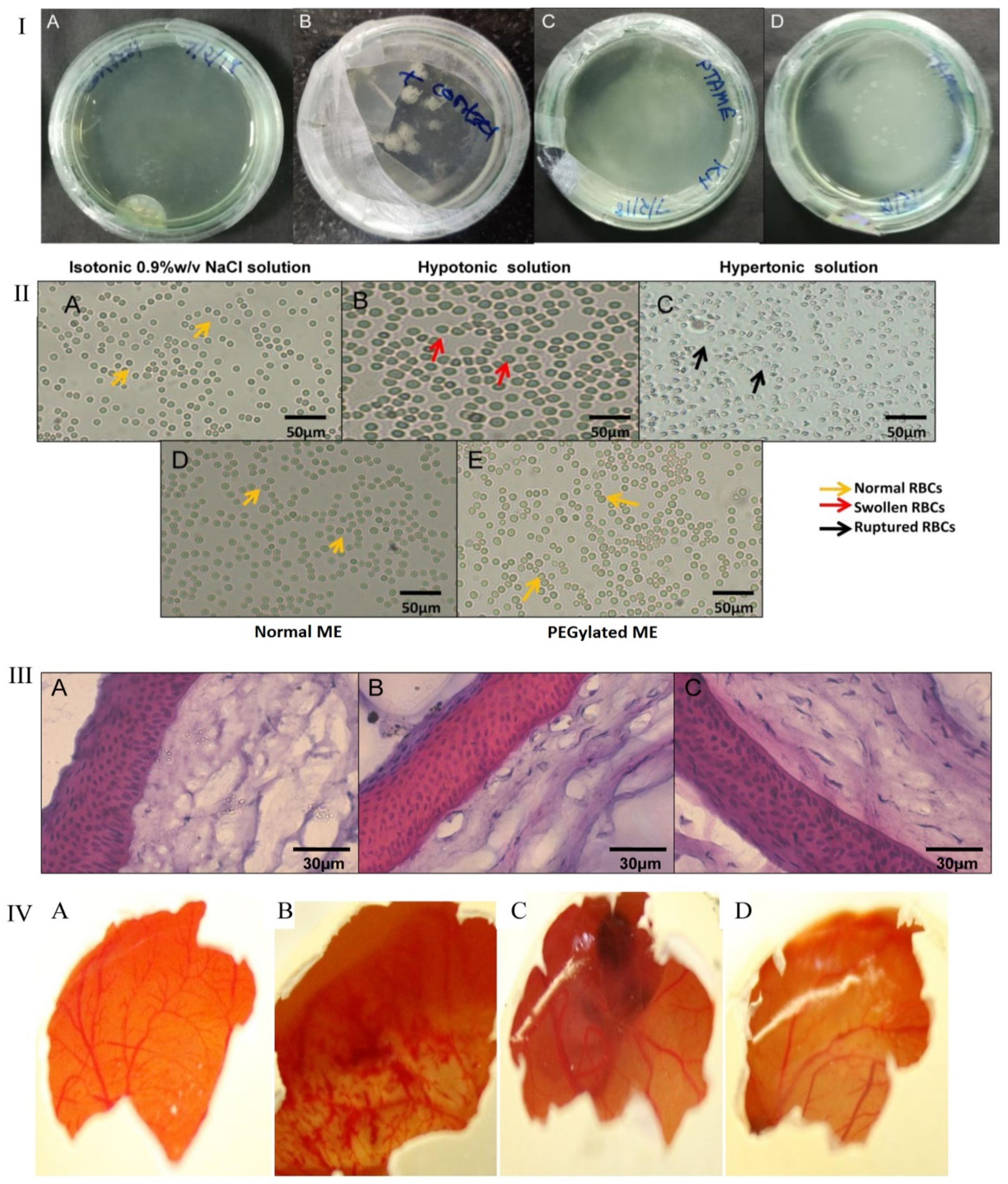
Figure 2. (I) Culture plate’s sterility test on incubation with (A) saline solution, (B) positive control, (C) PEGylated ME, and (D) normal ME; (II) tonicity evaluation, RBCs treated with (A) saline solution, (B) hypotonic solution, (C) hypertonic solution, (D) normal ME, and (E) microscopy of PEGylated ME; (III) hematoxylin-and-eosin-stained corneal sections treated with (A) saline solution, (B) normal ME, and (C) PEGylated ME observed under a microscope; (IV) corneal hydration test. Images captured after 3 h of hen’s egg membrane treated with (A) saline solution, (B) NaOH solution, (C) normal ME, and (D) PEGylated ME. Modified from ref. [2] (https://pubs.acs.org/doi/10.1021/acsomega.9b04244, (accessed on 20 December 2021)).
Kalam et al., (2016) prepared and evaluated gatifloxacin efficacy in the anterior segment of the eye with respect to good corneal adherence and permeation of the drug and compared it with a conventional eye drop. The prepared ME used an oily phase as isopropyl myristate and a nonionic surfactant such as Tween 80, and Transcutol-P was used as cosurfactant while applying an aqueous titration technique. The formulation appeared to have uniform droplets, and size ranges varied from 51 nm to 74 nm, with a surface charge on ME recorded as 15 mV to 24 mV and optimum physicochemical features desirable for topical instillation. The optimized formulation improved stability and contact time, and resulted in a twofold improvement in bioavailability of the drug, compared with the conventional eye drop. Thus, the ME showed improved intraocular permeation and trans-corneal penetration, as well as preventing precorneal loss and improving absorption of gatifloxacin in the anterior segment of the eye [3].
Perminaite et al., developed a novel royal jelly containing 10-hydroxy-2-decenoic acid-based ME for ophthalmic delivery. Royal jelly obtained from natural worker honeybees has potential biological activities such as anti-inflammatory and antioxidant activities. The royal jelly ME prepared by oil titration method comprised royal jelly, Tween 80 as surfactant and Tween 20 as cosurfactant, an oily phase as isopropyl myristate, and water, characterized in vitro. Further, the ME was assessed for irritation in the rabbit’s corneal cell culture. The results demonstrated that ME droplet size was 67.88–124.2 nm, with a polydispersity index of <0.180. The10-hydroxy-2-decenoic acid release depended on the surfactant and cosurfactant ratio employed in the formulation. The cell culture test results indicated that ME was nonirritating [4].
3. Polymer Micelles
Nanomicelles are the amphiphilic self-assembling architecture of colloidal particles, with sizes varying from 10 nm to 100 nm, and consist of a hydrophilic head and hydrophobic shell. The nanomicellar delivery system is commonly used to dispense therapeutics into a transparent aqueous solution. It is a widely employed pharmaceutical vehicle for solubilizing hydrophobic drugs. The poor solubility of the drug is a limiting factor for formulating the ocular preparation because of subtherapeutic effects in ocular tissues, which comprises an amphiphilic surfactant or a polymer in the aqueous phase. The carrier system’s important key attributes include easy preparation techniques, high entrapment of the drug, loading, nanosize, and capability to encapsulate hydrophobic drugs and remain in their hydrophobic shell. The carrier system enabled protection against drug degradation and increased drug stability in the aqueous phase. Micelles can be used for the delivery of prodrug, drug–polymer conjugate, and polymer film for sustained release in the ocular system [5]. An illustrative novel therapeutic strategy in ocular drug delivery is shown in Figure 3.
Figure 3. Novel therapeutic strategy in ocular drug delivery.
Civiale et al., prepared Dex nanomicelles using copolymers of polyhydroxyethylaspartamide PHEAC (16) and pegylated PHEAC (16) for topical anterior segment drug delivery. The animal studies of Dex nanomicelles were performed in rabbit’s aqueous humor, and results depicted that drug-loaded micelles increased ocular availability of drug by 40%, compared with a Dex suspension. In an attempt to enrich the drug concentration in the posterior ocular tissue, a combination drug (Dex, voclosporin, and rapamycin) with mixed nanomicellar preparation (0.1% and 0.2%) was designed. The tissue distribution analysis after single drop administration indicated that nanomicellar preparation containing multidrug enabled therapeutic concentrations to be achieved in the posterior chamber of the eye. These findings suggest that nanosize micelle could evade the physiological barriers in the ocular region and efficiently deliver drug carriers to the posterior ocular tissues [6].
Junnuthula et al., developed self-assembled block-copolymers of polymeric micelles and polymersomes and investigated for physicochemical features, including interactions and retention with vitreous liquid. Furthermore, they performed in vivo study in rabbits for ocular kinetics via intravitreal injections. Their findings revealed that polymersomes retention prolonged in the ocular tissue and deposited more to the retina, as well as the optic nerve, in the head region [5].
Alami-Milani et al., designed Dex-encapsulated polycaprolactone–polyethylene glycol–polycaprolactone micelles, and an ex vivo permeation test indicated thatmicelles could potentially deliver the hydrophobic Dex in the ocular tissue [7]. Vaishya et al., (2014) developed and characterized Dex-loaded polymeric nanomicelles for the posterior segment uveitis treatment. Pertaining to this, a low-molecular-weight di-block copolymer was synthesized and evaluated in vitro for the formulation of critical micelle concentration and tested in ocular cells for toxicity. The nanomicelles size incurred 25–30 nm, with uniform distribution and a polydispersity index of 0.125. The permeation of drug-loaded nanomicelles was raised by 2-times across the conjunctival cell line and by 2.5-times across the excised rabbit sclera, compared with a drug suspension. Thus, nanomicellar preparation herein developed could achieve therapeutic levels in the posterior region of the eye after topical instillation [8].
The topical treatment of posterior uveitis is noteworthy but goes against conventional therapy to achieve therapeutic concentration. In a similar attempt, to maximize the drug concentration in the posterior eye segment, Nikita et al., designed an everolimus-loaded nanomicellar preparation using Soluplus®, a grafted copolymer of polyvinyl caprolactam–polyvinyl alcohol–polyethylene glycol (PVCL–PVA–PEG) for enhanced permeation and bioavailability in the ocular epithelia to treat the ocular uveitis. The nanomicelles had a size of 65.55 nm and low CMC (7.2 µg/mL). The surface analysis was found to be uniform, spherical, and smooth. The drug entrapment was high, and the release profile was sustained, compared with adrug suspension. The permeation study in the cornea of goat mucosa suggested higher permeation across the cornea than drug suspension. Further, the higher drug permeation of nanomicelles was confirmed by confocal microscopy. Overall, the outcomes of the study clearly pointed to higher drug access and enhanced bioavailability of everolimus-loaded nanomicelles and revealed that these nanocarriers could be promisingly employed in the treatment of ocular uveitis [9].
Further, Patel et al. prepared Dex-loaded nanomicelles of polyoxyl 40 stearate and polysorbate 80 and characterized in vitro for solubility, critical micellar concentration formation, micelles size, zeta potential, surface morphology, drug release, and efficacy in an animal model. The ocular drug tolerance and drug distribution in ocular tissue were investigated in an animal model after single and multiple dosing. The developed nanomicelles containing Dex (0.1% w/v) showed micelles size of 13.3 ± 0.4 (placebo) and 14.5 ± 0.4 nm (drug-loaded nanomicelles). The TEM image observed a spherical, stable, and uniform micelle size. The animal testing revealed no inflammation, redness, or irritation when compared with control. The drug concentration was sufficient to exert a therapeutic effect in the cul-de-sac cavity after topical use in the rabbit’s eye. The generated novel nanomicelles enabled the solubilization of 0.1% Dex hydrophobic core and potentially delivered the drug in the posterior segment of the eye for treatment of posterior uveitis [10].
For active targeting based on peptide transporter-1, Xu et al. designed nanomicellesfor ocular delivery. They prepared chitosan oligosaccharide–valylvaline–stearic acid (CSO–VV–SA) nanomicelles and castor oil-40/octoxynol-40 (HCO-40/OC-40) mixed nanomicelles. The in vitro cytotoxicity assay produced no significant difference in human corneal epithelial cells (HCEpiCs) and conjunctival epithelial cells (HConEpiCs). The inhibitory test confirmed the active transport of CSO–VV–SA nanomicelles through the chosen transporter. The fluorescence study confirmed the active transport of CSO–VV–SA nanomicelles by PepT-1 in the posterior segment via the conjunctiva. The animal study demonstrated precorneal retention of Dex from both nanomicelles of more than 3 h. The results indicated that CSO–VV–SA nanomicelles could be novel carriers, with promising testing potential in clinical applications [11].
4. Nanoparticles
NPs are colloidal nanoparticulate carriers whose size generally varies in the range of 10–1000 nm. For ocular drug delivery, NPs comprise a mixture of protein, lipids, and or polymers derived from synthetics such as PLGA, polylactic acid (PLA), albumin, alginic acid, chitosan–alginate, and polycaprolactone. NPs improved the ocular passage of hydrophobic drugs by disrupting superficial ocular barriers and granting systemic access to medicaments from specific sites [12]. The drug-encapsulated NPs have desirable biological characteristics related to the enhanced ocular residence time from the dosage form, reduced toxicity, and increased drug penetration capability deeper to the ocular tissue, and concomitant with reduced drug loss from the precorneal spaces due to rapid tear fluid turnover [13]. NPs are promising carriers of drug candidates in ocular delivery, owing to their small scale, little eye irritation, and prolonged drug release, hence the reduction in dosing frequency. Due to easy elimination from the precorneal pocket, as seen generally with aqueous drug solutions, NPs administration is designed with a mucoadhesive feature likely to aid for more time in the precorneal chamber. For this, chitosan polyethylene glycol (PEG) and hyaluronic acid are preferably used to increase the pre-corneal residence time of drug-loaded NPs [14].
NPs with chitosan polymer are widely researched for ameliorating drug concentrations in the precorneal cavity by improving the ocular residence time. The chitosan is positively charged, which binds effectively with the negatively charged surface of the cornea and thus improves corneal residence time and minimizes the precorneal clearance. It was illustrated that natamycin chitosan/lecithin NPs improved ocular bioavailability by 1.47 fold and reduced precorneal clearance by 7.40 fold, at a low dose, and reduced frequency of instillation in rabbit’s eye, compared with a marketed suspension [15]. Musumeci et al., reported that melatonin-encapsulated PLGA–PEG NPs were effective in lowering intraocular pressure (IOP), compared with melatonin PLGA NPs and drug solution of an equivalent concentration in the rabbit’s eye. It was indicated that the decreased surface charge of PLGA–PEG, compared with PLGA alone, well accorded and enhanced interaction of NPs with eye surface, resulting in better hypotensive outcomes for a prolonged time [16].
Zhang et al., instilled Dex in rabbit’s eye through intravitreal injection and investigated pharmacokinetics and ocular tolerance of the drug from PLGA NPs. The results concluded that DEX-encapsulated NPs showed sustained release of the drug for50 days. The vitreous humor reported constant drug levels (3.85 mg/L) for 30 days. The results implied that Dex NPs via intravitreal injection provided sustained release in the posterior segment of the eye [17]. Chi et al., studied hybrid NPs and nanosheets for enhanced cellular uptake in the ocular tissues, using peptide transporter-1. They prepared and characterized nanocarriers in vitro. Both nanosheets and hybrid NPs indicated the sustained type of drug release in vitro and enhanced the precorneal retention in vivo, but hybrid NPs showed higher permeability in vitro than nanosheets. Furthermore, a cellular uptake study on HCEpiCs and ARPE-19 cells showed endocytosis based on actively transported PepT-1 and higher drug internalization, both from hybrid NPs and nanosheets. Thus, it was concluded that hybrid NPs are promising carriers for ophthalmic instillation in the mid-posterior region, whereas nanosheets are ideal for ocular diseases [18].
Yu et al., developed several Dex–glycol chitosan (Dex–GCS) conjugate by chemical synthesis and characterized for UV–Visible spectroscopy, infrared spectroscopy, and X-ray diffraction technology. The conjugate self-assembled into NPs with a size range of 277–289 nm and a positive surface charge of +15 mV. The particles were ascertained as spherical via transmission electron microscopy (TEM). Moreover, mucoadhesive properties of Dex-GCS NPs with varying concentrations of mucin were evaluated in vitro. Dex release in phosphate-buffered saline (PBS, pH = 7.4) expressed progressive drug release till 8 h and then reached plateau upto 48 h. The cytotoxicity against L929, HCEC, and RAW 264.7 cells of the formulation was tested after incubation of 24 h and showed similar efficacy to Dex sodium phosphate (Dexp) in lipopolysaccharide (LPS)-activated RAW 264.7 macrophages. More interestingly, the developed Dex-GCS NPs established effective ocular tolerance and precorneal retention, compared with an aqueous preparation, indicating that the self-assembled Dex-GCS NPs appear to be an anticipated system for ocular therapeutic delivery [19].
5. Nanoparticulate Targeting in Retinoblastoma (RB)
Retinoblastoma is encountered during childhood, and the incidence rate is more prevalent in children under the age of 5 years. The survival rate of the cancer is high but may lead to severe complications such as vision loss and even death if not diagnosed and treated timely [20]. After detection of cancer, medical intervention relates to chemotherapy, radiotherapy, and or surgery, which are meant to improve patient survival. The NP-based drug delivery investigated in RB led to improved drug delivery in the posterior eye segment and also increased the intravitreal half-life (t1/2) of chemotherapeutic agents with potential outcomes in retinal cancer [21]. A nanoparticle targeting based on rationale design essentially incorporates functionalized moieties or ligands for potential cellular uptake, and cellular internalization of therapeutics has been reported in several publications [22][23][24][25]. Several types of conjugating agents—namely, epithelial growth factor receptor (EGFR), folic acid, transferrin, cell penetration peptide, and proteins, are used for surface functionalization processes, depending upon the dominancy of specific receptor to the target site [26]. Further, it has been observed that the surface alteration of nanocarrier using polyethylene glycol (PEG) improved the NP uptake due to colloidal stability, reduced protein adsorption, and less opsonization, thus improving the intravitreal transport to target cells [27]. Sims et al. designed functionalized melphalan-loaded poly(lactic-co-glycolic acid) (PLGA) NPs to increase the intravitreal drug delivery through positive cell association and improved efficacy in retinoblastoma cells. They compared the cell association potential and efficacy in retinoblastoma cells surface-modified PLGA NPs with unmodified NPs. They observed prominent cell association, cell internalization, and enhanced efficacy with surface functionalized MPG-NPs after 24 h of administration, compared with unmodified NPs. In another study, topotecan-bearing mesoporous silica NPs with folate conjugation had enhanced drug efficacy in RB treatment. The nanosized particles demonstrated sustained drug release and superiorcell uptake in Y79 RB cells, compared with nontargeted NPs [28].
6. Nanosuspensions
Using conventional techniques to formulate hydrophobic substances is highly challenging. The application of nanotechnology in formulating hydrophobic drug substances such as nanosuspension is desirable to address the problem associated with drug molecules. Nanosuspension is a colloidal carrier of dispersed drug substances of submicron particle size, which are, in turn, stabilized by formulation additives such as surfactants or polymers. In ocular drug delivery, the method offers many benefits such as ease of sterilization in formulating the eye drop, minimizing ocular irritation, improving precorneal residence, as well as enhancing ocular drug absorption. Many studies well reported the improved glucocorticoids absorption via ocular drug delivery. Glucocorticoids such as Dex, prednisolone, and hydrocortisone have been widely suggested for the therapeutic modality of inflammatory conditions in ocular tissues of the anterior segment. However, the conventionally established treatment using these drugs needs multiple dosing, resulting in large cumulative doses that may further lead to complicationssuch as cataracts, optic nerve damage, or glaucoma. Kassem et al. successfully developed glucocorticoids (Dex, prednisolone, and hydrocortisone) nanosuspension, and the formulation was effective in reducing the intraocular pressure in rabbit’s eye [29].
Recently, Yan et al., compared mucus-penetrating particles (MPPs) and cationic NPs suspension having cyclosporine A (CsA) in terms of ocular bioavailability. Researchers further clarified the mucous permeation capacity of MPPs and mucous-retaining capability of cationic NPs, although both preparations were capable of prolonging the ocular residence time of the drug on the eye surface. Both cationic nanosuspensions and MPP nanosuspensions (drug core) were prepared by applying an antisolvent precipitation technique. The X-ray analysis revealed that CsA was in an amorphous state in both formulations. The in vitro mucoadhesion analysis showed that cationic nanosuspensions interacted 5.0–6.0 times higher with pig mucin, compared with MPP nanosuspensions (drug core). The permeation study on drug-core MPP nanosuspensions indicated that apparent permeability (Papp) value was 5.0–10.0 times greater than cationic nanosuspensions. The in vivo ocular bioavailability showed CsA concentration in cationic and MPP (drug-core) nanosuspensions were 13,641.10 ng/g and 11,436.07 ng/g, respectively, which was significantly greater than conventional nanosuspension (8310.762 ng/g). These results indicated that both cationic and MPP nanosuspensions were effective in delivering the CsA concentration (10–20 μg/g) to the anterior chamber using eye drops. Therefore, cationic nanosuspensions look promising, as they provided more ocular bioavailability than MPP nanosuspensions [30].
Boddeda et al., prepared flurbiprofen (FB)-encapsulated polymeric nanosuspension for enriching the bioavailability in the ocular region. The nanosuspension was developed by a solvent displacement technique while optimizing the process variables—namely, drug and polymer ratios and aqueous-to-non aqueous solvent ratio, as well as their impact on formulation characteristics including size, drug release, and ocular tolerance. The developed nanosuspension showed a spherical particle shape, a particle diameter of around 100 nm to 200 nm, with surface charge ranging from +6.6 ± 2.2 to +19.0 ± 3.1 mV, and drug encapsulation was recorded between 54.67 ± 3.4 and 90.32 ± 3.2%. The nanosuspension underwent sustained release of drug (60%) over 12 h, compared with a marketed preparation (Flur eye drops). In vivo study of an animal model reported that it was nonirritant and safe, based on histopathological studies. The FB-loaded Eudragit nanosuspension proved to be safe, stable, and suitable for ocular use [31].
7. Liposomes
Liposomes are widely sought drug delivery carriers with vast applications in different areas of biomedical sciences, including topical applications. They are lipid-based spherical vesicles that have one or more cell membranes such as phospholipid bilayers encasing the aqueous phase and proved as promising drug delivery carriers for ocular disease therapy due to the enhanced ocular residence time for drug absorption. The size of the vesicle ranges between 10 nm and 1000 nm, depending upon the phospholipid layer:unilamellar vesicles (10–100 nm), bilamellar vesicles (100–300 nm), and multilamellar vesicles (>300 nm). In recent times, liposomal drug carrier remains a point of interest for ocular drug delivery. The liposome is an ideal drug carrier owing to its remarkable biocompatibility, high degradability, flexibility, nonimmunogenicity, nontoxicity, and being mimetic to cell membrane architect, enabling the encapsulation of both lipophilic and hydrophilic drugs and delivering the medicaments effectively in both anterior and posterior chambers [32]. Various studies investigated the liposomal drug delivery for improving dissolution, bioavailability, precorneal penetration, increased residence time, and targeted action [33][34][35].
Age-related macular degeneration (AMD) in the eye is a leading problem associated with the central region of the retina, i.e., the macula of the eye, which may lead to visual deficiency and at later stages to blindness. To improve the solubility and bioavailability of berberine hydrochloride (BBH) and chrysophanol (CHR) for the treatment of ocular diseases based on active biological response related to anti-inflammatory, antioxidative, and antiangiogenic effects, Lai et al. developed PAMAM-coated liposomes. The PAMAM-coated liposomes indicated considerable cellular permeability in the corneal cells and increased bioadhesion on the corneal epithelium of the rabbit model. The coated liposomes improved drug absorption and acted apparently as protection for the retinal pigment cells and also protected the rat’s retina after photooxidative injury. The formulation of liposome pointed to no side effects post investigation of ocular morphology in the rabbit. The cellular internalization of the developed formulation was investigated in HCEC cells after incubation of 24 h. As indicated in Figure 4a,b, PAMAM-coated coumarin (Cou) liposome showed stronger fluorescence intensity, compared with normal liposomal formulations, after 1 h of topical administration. The study suggests that PAMAM-coated Cou liposomes may significantly elicit the cell uptake of therapeutics from carrier systems, compared with normal liposomes [34].
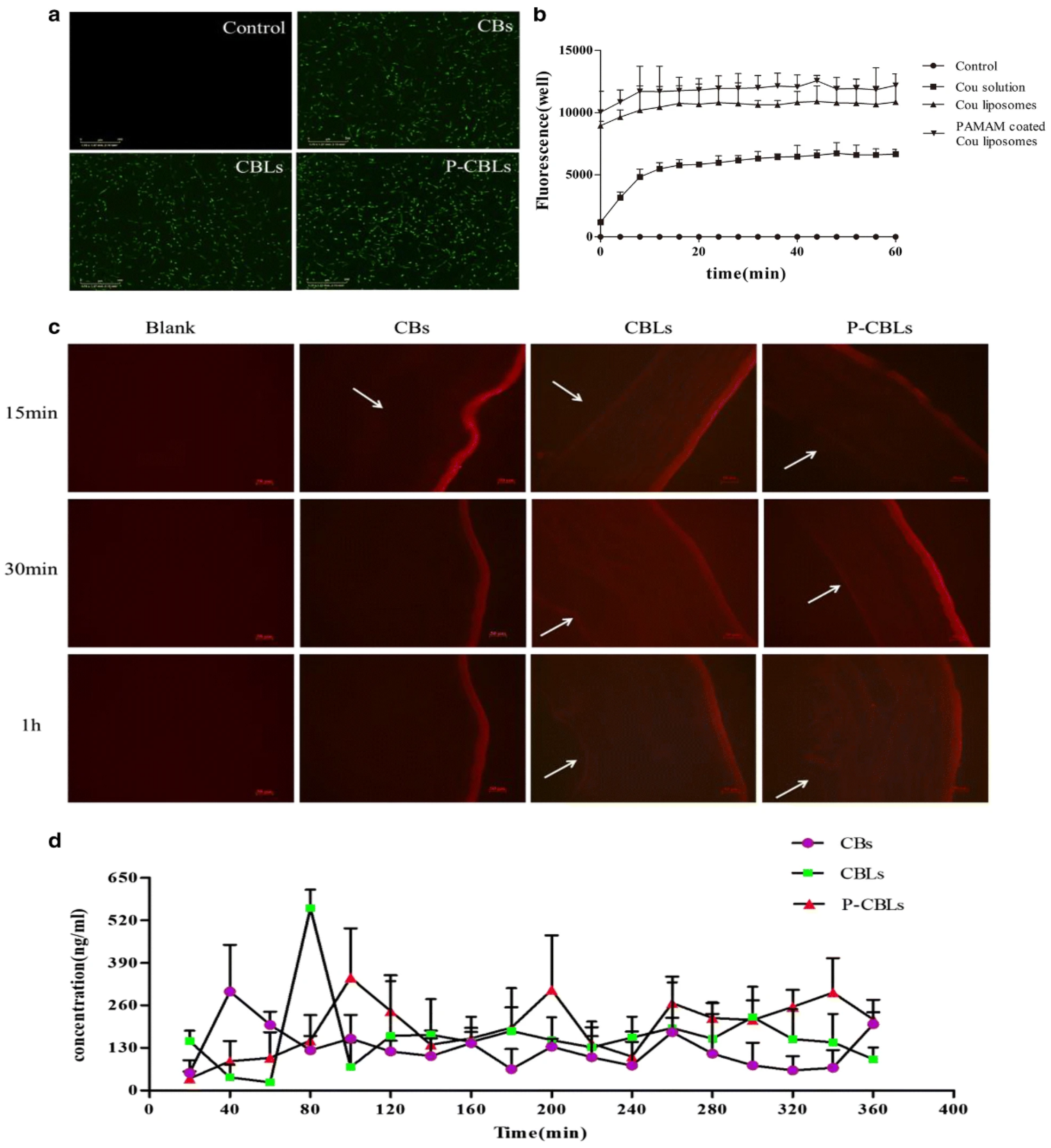
Figure 4. Investigation of liposomal effectiveness in ocular drug delivery: (a) the fluorescence images of different formulations with Coumarin (Cou) using cell analyzer. The cellular uptake after 24 h of human corneal epithelial cells (HCECs) (scale bar = 300 μm); (b) formulations intake count; (c) the Nile red-stained formulation distribution images captured in cornea; the corneal endothelium indicated by arrow (scale bar = 50 μm); (d) in vivo pharmacokinetic parameters after topical instillation of different formulations. Permission under Commons Attribution 4.0 International License [34]. (http://creativecommons.org/licenses/by/4.0/, (accessed on 15 November 2021)).
The different preparations—namely, chrysophanol–berberine hydrochloride suspensions (CBs), compound liposomes (CBLs), and PAMAM-coated compound liposomes (P-CBLs) were used to examine the transcorneal permeability. Each of the formulations can similarly penetrate the corneal epithelium, as indicated by the fluorescence intensity for 15 min initially after topical instillation. As regards moving time, the concentration of CBLs and P-CBLs were more detected in the corneal epithelium, shown by high fluorescence intensity. High drug retention in the ocular tissue was also confirmed because of the lack of drug in tear fluid. Moreover, for CBs, the fluorescence intensity was diminished in the corneal endothelium, indicating that they are unable to permeate through the corneal epithelium (Figure 4c). A pharmacokinetic study was performed using formulation CBs, CBLs, and P-CBLs in the rabbit’s eye. Notably, the outcome of the study revealed that Cmax of BBH in the aqueous humor with instillation of P-CBLs and CBLs were 1.719 and 1.23-times greater than CBs. The bioactivity of BBH loaded liposomes was 1.33 times greater than BBH-Loaded CBs, whereas P-CBLs raised the bioactivity by 1.6343 times vis à vis CBs. Therefore, the PAMAM-coated liposomal system showed potential utility in treating complex ocular ailments [34].
Moreover, pharmacodynamic studies were conducted to investigate the therapeutic efficacy in the ocular region, with liposome formulations, in light-induced retinal tissue damaged rat’s model. The pentobarbital sodium (3%) was injected viathe intraperitoneal routeinto different groups of animals—normal saline, placebo liposome, CB, CBL, and P-CBL groups. Among these formulations, P-CBLs induced the highest protection in the reversal of retinal function in photo-exposed rats. Flash electroretinogram after 14 days by light damage of retinal tissue indicated a significant increase in b-wave responses in P-CBLs-treated rats, compared with other formulation groups, as shown in (Figure 5a). The normal-saline-group-treated rats kept their intact retinal vessels, and the background of the fundus was clearly seen. The rat vessels treated with the blank liposome group showed some severe manifestation in the fundus. The P-CBLs had no impact on the retinal blood vessels, but they improved the reflection area in the eye, as shown in (Figure 5b). Compared with the normal saline group, the blank liposome group decreased the numbers of outer nuclear layer cells and led to the thinning of the layer significantly. Adversely, histopathological examination of the eye section showed evidence of protective impression in the retina in P-CBL-instilled rats after ocular cell injury caused by the photo-oxidative process. The morphological analysis revealed clear layers of retinal structure and well-stained nuclear layers in P-CBL-treated rats (Figure 5c). The antioxidant assay showed P-CBLs were highly potent in reducing ROS levels as per the relative fluorescence ratio, compared with CHR and BBH, as shown in Figure 5d,e [34].
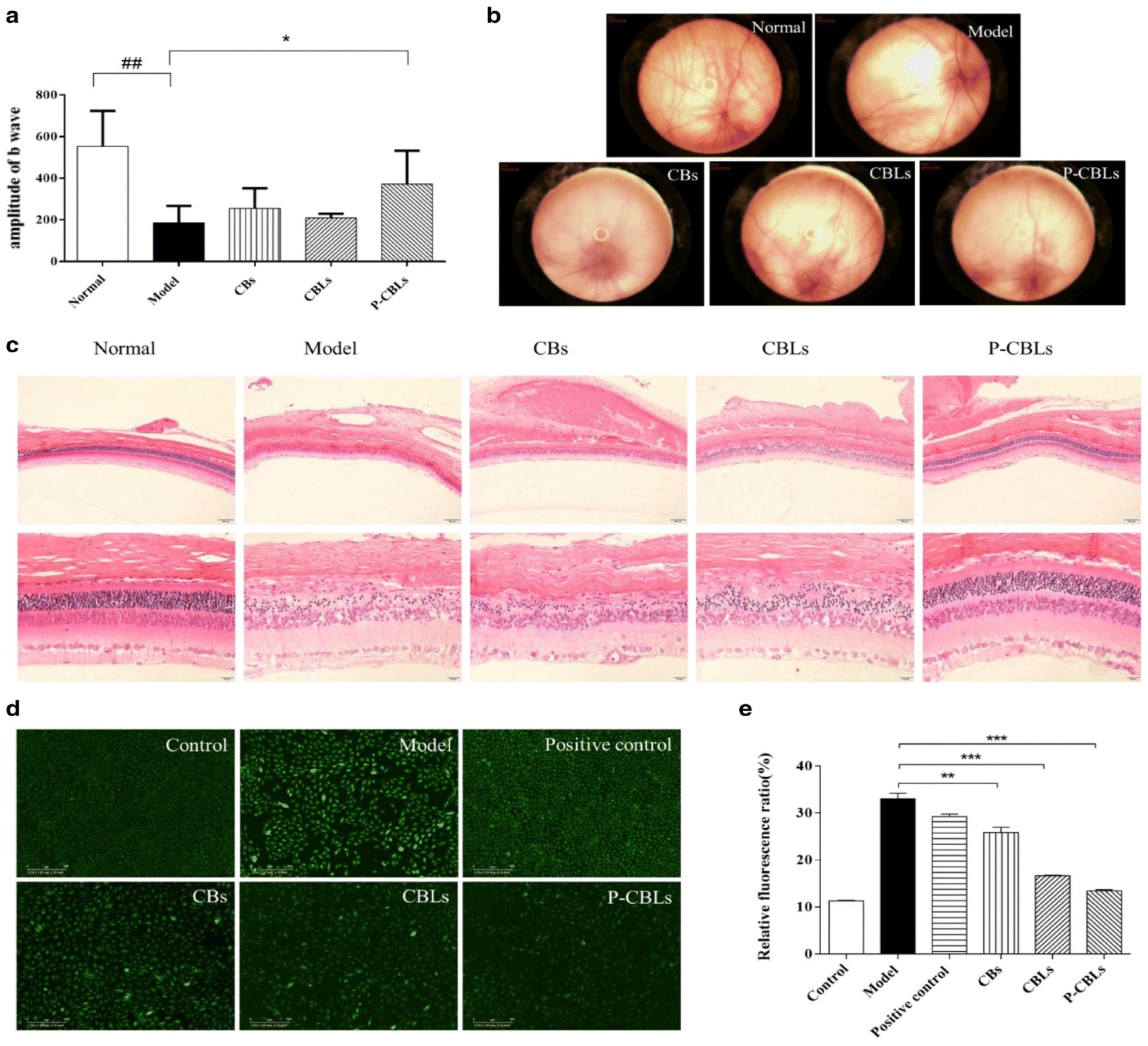
Figure 5. Effective drug delivery of liposomes into posterior ocular segment. Protection against photo-oxidative retinal damage: (a) the b-wave amplitude alteration following topical administration of different formulations for up to 14days; (b) retinographic images of various formulations treatment; (c) the protection efficacy of various hematoxylin-and-eosin (H and E)-stained formulations in the retina (scale bar = 20 μm); (d) images of in vitro anti-ROS efficacy taken with a long-term real-time dynamic live cell imaging analyzer: (e) ROS levels of various formulations. Data are expressed as mean ± SD (n = 3). * p < 0.05, ## p < 0.01, ** p < 0.01, *** p < 0.001. Permission under Commons Attribution 4.0 International License [34]. (http://creativecommons.org/licenses/by/4.0/, (accessed on 15 November 2021)).
Moreover, the ocular irritation study in rabbits was performed using the same formulation with equivalent drug doses such as CBs, CBLs, and P-CBLs, and the ocular surface was analyzed using the Draize eye test. The tissue histology analysis revealed that the cornea, iris, and conjunctiva were safe, and no tissue damage was seen in thegroup after 14 days of instillation of P-CBLs (Figure 6a). The stained ocular surface with 0.5% sodium fluorescein observed under a silt lamp and camera showed no edema or injuries, demonstrating the safe and protective nature of P-CBLs (Figure 6b) [34].
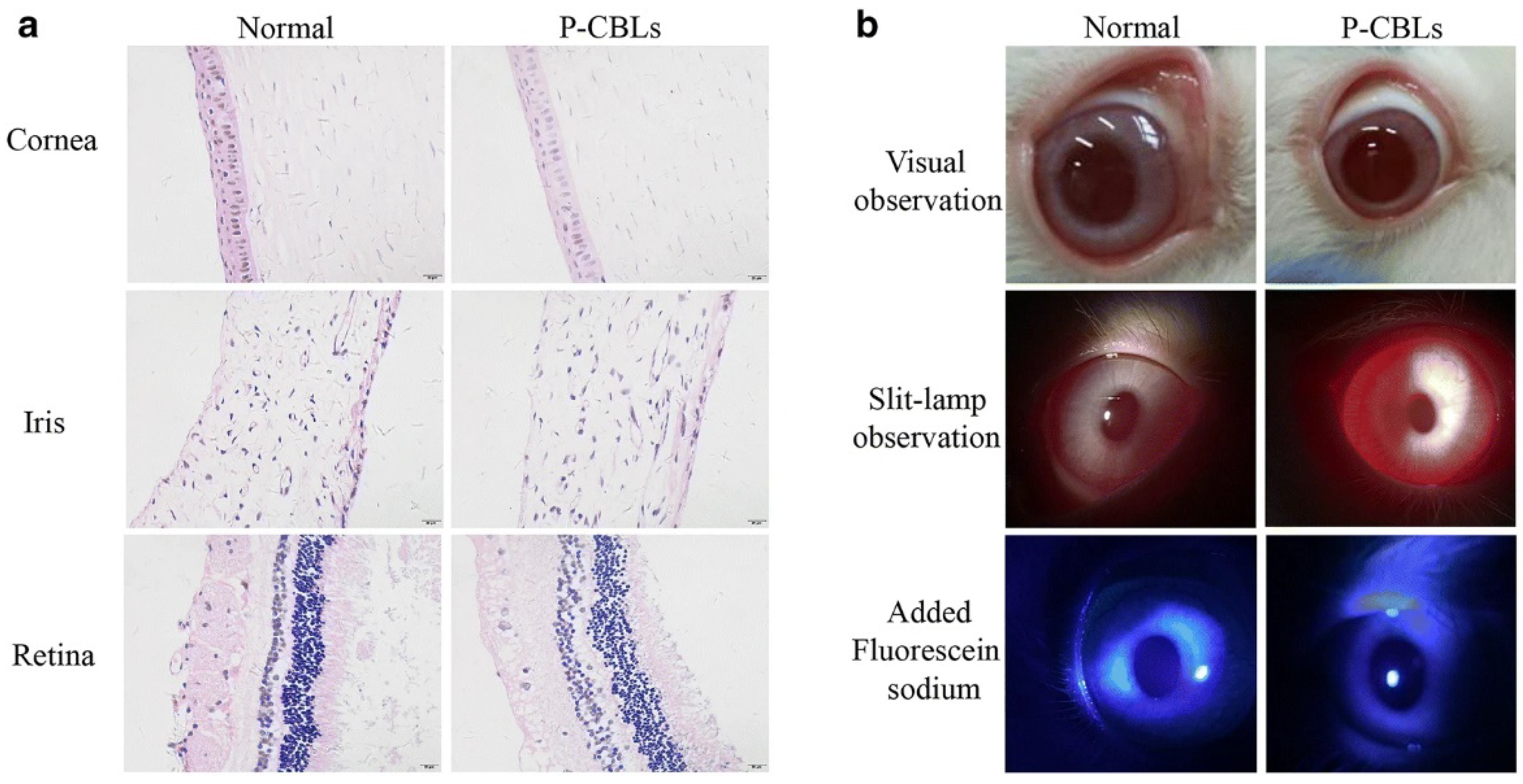
Figure 6. (a,b) Ocular irritation studies: (a) typical histological image of formulation, P-CBLs after instillation for 14 successive days (scale bar = 20 μm); (b) ocular surface examination using a silt lamp and camera, post staining with 0.5 % sodium fluorescein. The ocular irritation studies manifested no injuries or abnormalities in either part of the cornea, conjunctiva, or iris of the eye (a). The 0.5% sodium fluorescein stained ocular surface observed under a silt lamp and camera found no edema or injuries, which further substantiated the safe and protective nature of P-CBLs (b). Permission under Commons Attribution 4.0 International License [34].
A study led by Natarajan et al., investigated a latanoprost liposomal preparation for delivery to the anterior segment of the ocular tissues. The instillation of single liposomal formulation via subconjunctival injection in rabbit’s eye brought forth sustained lowering effect of IOP for upto 50 days, which is comparable to the conventional eye drop formulation. Cationic liposomes experienced better drug delivery efficacy in the posterior ocular segment, compared with anionic or neutral liposomes, by using positively charged lipid or mucoadhesive, there by improving ocular residence time of the drug. For instance, stearylamine and didodecyldimethylammonium bromide are generally employed in designing cationic liposomes [36].
To improve the antibiotic efficacy in topical instillation dos Santos et al., encapsulated besifloxacin into liposomes with additives as positively charged amines and investigated the impact of these charges on the drug diffusion process in two approaches—namely, iontophoresis and passive diffusion. The authors hypothesized that the charge present on the liposome surface could enhance the burst release due to electromigration upon application of electricity and improve the penetration efficiency and residence time of formulation. Herein, liposomes prepared by using phosphatidylcholine (LP PC) or phosphatidylcholine and spermine (LP PC:SPM) were stable, indicating the mucoadhesive property and that they were compatible withthe ocular tissues. Furthermore, electron resonance spectroscopy exhibited that drug and excipients incorporated in liposomal preparation did not interfere with membrane fluidity, structural integrity, and stability in the iontophoretic state. The liposome (LP PC) showed a mean diameter of ~177 nm and zeta potential of −5.7 ± 0.3 mV, and for liposome (LP PC:SPM), the mean diameter and surface charge were ~175 nm and +19.5 ± 1.0 mV, respectively. The minimum inhibitory concentration (MIC) and the minimal bactericide concentration (MBC) of the developed liposomes investigated for P. aeruginosa indicated lower MIC and MBC than the marketed preparation (Besivance). Both formulations showed the same efficacy, and surface charge incorporation on liposomes was not beneficial in iontophoretic therapy. On the other hand, as investigated in an ocular model in vitro, passive diffusion/penetration of a drug that simulates the tear fluid remains challenging in passive delivery of the formulation due to ocular resistance to the formulation. The result anticipated that liposomes LP PC:SPM expressed higher drug penetration than the marketed preparation, Besivance. Therefore, besifloxacin-loaded positive (+) liposomes ameliorated the passive delivery of drug upon topical instillation and could be considered a novel approach to enhance the ophthalmic disease therapy [33].
Rajala et al. examined the utility of liposome–protamine–DNA complex (LPD) in gene delivery through a subretinal manner. A biomimetic virus was designed to havemodifications in cellular and signaling peptides for the delivery of retinal pigment epithelium protein 65 (Rpe65) gene for eye disease treatment in mice. Rpe65 is a key enzyme that controls the photochemical 11-cis-retinal and helps to see objects. The results indicated that modified liposome showed effective Rpe65 gene delivery in a specific and further alleviated long-term expression of the Rpe65 gene in Rpe65 knockout mice, resulting inthe rectification of blindness in vivo [37].
In another study, acyclovir encapsulated in positively (+) and negatively (−) chargedliposomes were fabricated using stearylamine (cationic) and dicetylphosphate (anionic) charge-inducing agents. The drug concentration from positively charged liposomes in the cornea of the rabbit’s eyewas higher than the liposomes of negatively charged vesicle and plain acyclovir when administeredviatopical instillation after 2.5 h. The observed concentration drug in the cornea for the drug solution, andpositively and negatively charged liposomes were 253.3 ± 72.0, 1093.3 ± 279.7, and 571.7 ± 105.3 ng/g. The increase in drug absorption from positively charged liposomes was 2-times higher than negatively charged liposomes and 5-times higher than drug solution, indicating that positive surface liposomes havea great affinity with the negatively charged corneal surface, which may be ascribed to electrostatic interaction, thereby increasing the ocular residence time and drug absorption [38].
In the posterior segment, drug delivery of liposomal preparation is to be more concentric on elating the t1/2 of the drug by foreshortening the fluid clearance from vitreous humor and protecting degradable molecules viz., peptides and nucleotides, and furnishing sustained release of the drug. Referring to this, liposome enabled the increase influconazole t1/2 in the vitreous humor of rabbit eye approximately 8-times higher than plain drug [39].
In a similar study, a tacrolimus liposome was prepared for effective therapy of uveoretinitis. After i.v. administration of tacrolimus-loaded liposome, the concentration of drug in the vitreous humor level heightened to >50 ng/mL and showed sustained release behavior for 14 days. Thus, the tacrolimus liposomal formulation was proved more efficacious in limiting uveoretinitis, compared with the drug solution and also overcome the toxicity caused inside the retinal cells [40]. Among alarge number of liposomal formulations in the preclinical and clinical phases, few of them are commercially available such as Visudyne® and Tears again® used for the treatment of ocular diseases. Visudyne®, a liposomal preparation having verteporfin as a photosensitizer, is applied in photodynamic therapy for the growth of new blood vessels in choroidal cellsassociated with macular degeneration (age related), ocular infection, and myopia [41].
8. Dendrimer
Dendrimers are nanoscale, star-shaped multibranched structures comprising polymeric chains. They are accessible in various molecular grades with terminal end positions of –NH2, OH, and –COOH groups. The end position functional moiety may be used to functionalize with various ligands or targeting moieties. The multibranched dendrimer may allow a large number of lipophilic or hydrophilic drug moieties to become entrapped. Recently, using dendrimer as a carrier for delivering a drug through different routes in the ocular cavity has been reported, with a promising outcome of PAMAM dendrimer.
Vandamme et al., employed the utility of PAMAM dendrimers as ocular drug delivery vehicles of pilocarpine nitrate and tropicamide delivery in glaucoma miotic and mydriatic activity. The ocular retention time of PAMAM solutions, fluorescein saline, and fluorescein in carbopol solution (0.2% w/v) was investigated in the rabbit eye. The average ocular residence period of PAMAM and carbopol solution was accounted higher than the normal saline. Thus, dendrimers as ocular vehicles were suggested as desirable alternatives for enriching ophthalmic residence time and improving ocular drug availability, with better outcomes in the ocular therapy. For instance, pilocarpine nitrate- and tropicamide-loaded PAMAM dendrimers showed prominent miotic and mydriatic activity when administered in albino rabbits [42].
To overcome corneal inflammation and reduce dosing frequency, Soibermanet al., explored Dex-loaded hyaluronic acid crosslinked G4-PAMAM dendrimer gel for subconjunctival injection as a potential ocular delivery approach for sustained release and increased absorption of D-Dex. The therapeutic efficacy of the formulation was tested on a rat model. Herein, the fluorescently labeled dendrimers (D-Cy5) loaded in the gel were investigated for D-Cy5 release in vivo. The D-Cy5 was specifically released in the target inflamed tissue and remained confined to the corneal macrophages in the infected rat. Further, improvement in inflamed tissue of the cornea, corneal clarity, and reduced neovascularization by subconjunctival application of D-Dex gels were clinically proven over a period of 2weeksin comparison with the freeDex. The outcomes of the study established that D-Dex dendrimer was more effective in weakening the corneal inflammation than freeDex, probably through inactivating macrophage and cytokines expression (pro-inflammatory). The developed injectable gel of D-Dex could have potential as a treatment of inflammatory disorders in the ocular tissues related to keratitis, dry eye syndrome, as well as postsurgical problems [43].
Several drugs have been investigated in polyamidoamine dendrimers as novel platforms for ophthalmic drug release in the aqueous solution. Herein, the authors developed a fast-dissolving dendrimer-based nanofiber (DNF) based on dendrimer as a vehicle for topical drug delivery of brimonidine tartrate (BT) in glaucoma treatment. The drug release kinetics and safety concerns of the nanofiber were securely investigated both in vitro and in vivo and showed zero toxicity at therapeutic dose in cultured cells and no irritation caused in the normotensive rat model. The intraocular pressure post administration of a single dose having equivalent amounts of the drug in DNF and BT solutions was measured the same. The DNF indicated significant improvement in efficacy, compared with the BT solution, observed for 3weeks after once-daily dosing suggested that dendrimer nanofibers could act as alternatives for effective drug delivery used as eye drops for glaucoma therapy [44].
9. In Situ Gel
Hydrogel is a crosslinked polymeric system that has wide applications in medical sciences including drug delivery and tissue engineering. In ocular therapy, hydrogels are used as promising carriers for drug delivery in a cul-de-sac cavity on account of biocompatibility and their capability to hold both hydrophilic and lipophilic drug-loaded systems, protecting them for an adequate amount of time. Hydrogel acts as a drug depot, on-demand drug release, and tunable cargo could maintain a therapeutic window, thus enhancing drug absorption [45]. The polymeric structure can hold a large amount of water and or biological fluid in a swollen state. In situ hydrogel is a polymeric solution in an aqueous medium that has phase transition characteristics of sol-gel via physicochemical crosslinking, resulting in the formation of a viscoelastic gel. The gel-forming capacity can be enhanced by alteration in heat or temperature, medium pH, and ions or may be developed through UV irradiation. The stimuli-responsive thermosensitive gel is widely explored for a number of therapeutics in ocular drug delivery [46]. There are several thermo-gelling polymers that have been accounted for in the literature for ocular delivery, including poloxamers, poly (N-isopropylacrylamide), copolymers of polycaprolactone, polyester block copolymers polyethylene glycol, poly (lactide), and glycolide, as well as chitosan. The thermogelling polymer at room temperature remains in a liquid state but can solidify into gel post injection at a physiological temperature [47].
Phua et al., rationally developed a preparation to outweigh the limitation of poor bioavailability in ophthalmic treatments. The preparation comprised thermosensitive hydrogels of pluronicF-127 meant, to strategically enhance the bioavailability by improving ocular residence of drug-encapsulated nanoliposomes dispersed in thermosensitive hydrogels. They prepared a depot preparation of nanoliposomes for subconjunctival injections. Senicapoc-loaded nanoliposomes showed sustained release from nanoliposome and hydrogel preparations. The in vivo study in Sprague Dawley rats showed a 12-fold increase in ocular residence time, with 24% hydrogel preparation for 1 h, compared with 5 min for free liposomes, observed with fluorescence measurement. A pharmacokinetic study on flushed tears revealed that hydrogels enabled drug retention for a long time, compared with a viscous preparation (1 h), and drug concentration could also be detected in conjunctival tissues within 24 h post injection [48].
Tacrolimus (TAC) is a hydrophobic drug. The marketed formulation of TAC as eye drop causes poor drug retention in precorneal space, low aqueous stability, and pulse kinetic pattern, overall leading to less drug absorption. Sun and Hu developed TAC-loaded SLN in situ gel (TAC-SLNs ISG) for ocular drug delivery. The optimized formulation was characterized for in vitro performances including drug release properties. The pharmacokinetic and pharmacodynamic studies were also performed, to investigate the impact of formulation in comparison with a drug suspension. The probe sonicated particle of TAC-SLNs ISG had a particle size of 122.3 ± 4.3 nm, and the same was changed nonsignificantly in situ gel. The viscosity of the formulation resulted in pseudoplastic flow. The gelation temperature of the developed gel was 32 °C, and a marked rise in viscosity was observed and formed a rigid gel at a higher temperature. In vitro study illustrated the sustained release of the drug from TAC-SLNs ISG. An in vivo pharmacokinetic study showed that eye drops achieved Cmax 4657.7 ng/mL within 30 min; on the other hand, TAC-SLNs achieved Cmax 1892.6 ng/mL in 30 min, and TAC-SLNs-ISG had the highest concentration of 2132.3 ng/mL within 2 h. The lower concentration early on from such formulation was probably due to the sustained release effect. The AUC0–t of TAC-SLNs-ISG and TAC eye drops were 590,355.9 and 222,382.5 ng·min/mL, i.e., 2.65-folds higher for TAC-SLNs-ISG than for TAC eye drops. The data clearly point to the superiority of TAC SLNs-ISG, compared with eye drops [49].
In 2020, Noriakiet al., developed tranilast NPs (ophthalmic TL-NPs formulations) for enhanced drug penetration into the ocular tissues. They designed in situgel, integrating TL-NPs with methylcellulose (MC, 0.5–3%), to improve the ocular residence time of the drug. TL-NPs preparation was fabricated using the bead mill method, and the resulting particle size was ~93 nm. An animal study using rats showed that the concentration of drug in the lacrimal fluid was enhanced when the preparation was developed using the MC (0.5–1.5%) concentration. The drug deposition in the cornea and conjunctiva and the anti-inflammatory effects of TL were observed in rats post instillation, with an ophthalmic TL-NP preparation. The optimized formulations of TL-NPs gel with MC (0.5–1.5%) ensured a long residence and improved contact time in the conjunctiva, compared with a formulation using TL-NPs with 3% MC [50].
10. Nanocapsules and Nanospheres
Depending upon the structural integrity of polymer NP, this is categorized as nanocapsules or nanospheres. Polymeric nanocapsule has been widely examined and interest in its use as a drug delivery carrier has increased in recent years due to unique nanostructure that comprises an outer part of a polymeric shell and inner part as a liquid or solid core [51]. Katzer et al., prepared prednisolone-containing nanocapsules (NCs) by interfacial deposition technique using polycaprolactone or Eudragit® RS100. The prepared NCs were subjected to physicochemical characterization in vitro for particle size distribution using laser-based spectroscopy and size-tracking analysis. Furthermore, cytotoxicity and ocular irritation were determined on the epithelial cell line of the cornea and chorioallantoic membrane of a rabbit model. NCs were reported to have mean sizes between 100 nm and 300 nm, and prednisolone entrapment was 50%. The NCs showed controlled prednisolone biexponential release for upto 5 h. Both formulations were safe in the CAM test due to being nonirritant and showed no cytotoxicity in corneal epithelial cells in the rabbit. The prednisolone nanocapsule was successfully developed for the first time, for application as eye drops in the treatment of eye inflammation [52].
Rebibo et al., (2021) used tacrolimus as a model drug to treat eye inflammation. Bearing in mind the rapid drug expulsion from the carrier system, a critical challenge in ocular drug instillation, they developed tacrolimus-encapsulated nanocapsules (NCs) for ocular instillation. The developed formulation was assessed for stability and efficacy under different experimental conditions. The characterization of the NCs showed the uniform size, and encapsulation efficiency was high, upto 80%. Furthermore, the lyophilized product showed good stability as per ICH guidelines for 18 and 3 months under long-term and accelerated stability conditions. Moreover, drug-loaded NCs did not show any irritation in the rabbit eye post single and multiple-dose schedules. In addition, ex vivo study of drug penetration on the porcine cornea, as well as pharmacokinetics analyses in various eye compartments of the rabbit, showcased the high retention and permeation of drug from NCs into the anterior chamber of the eye, compared with plain drugs present in the base. An animal study in rats also revealed high tacrolimus concentration in the eye. The designed tacrolimus NC system tested on a murine model of keratitis showed a significant reduction in several inflammatory markers, leading to reduced inflammation in the anterior chamber. The outcomes of the study showed that NCs as eye drops provided clinical and histological effectiveness, chiefly in the inflammation of the posterior eye chamber in the murine model and experimental auto-immune uveitis [53].
Bevacizumab has been employed in ocular therapy in age-related macular degeneration (AMD) in many countries, and due to their short biological t1/2, multiple intravitreal injections are required. Li et al., prepared PLGA and PEGylated nano- and microspheres using bevacizumab, with an aim to improve the drug retention time in the ocular cavity. The release profile of the developed formulation evaluated in vitro showed bevacizumab release in a sustained fashion over a period of 3 months [54].
Robinson et al. prepared and evaluated epidermal growth factor receptor (EGFR), tyrosine kinase inhibitor (TKI), and AG1478-loaded PLGA microspheres and nanospheres meant for intravitreal injection in rats through an optical nerve crush injury model. In vitro characterization of microspheres and nanospheres revealed particle sizes of ~2.6 μm and ~360 nm. After intravitreal injection, the optic nerve regenerated within two weeks. Moreover, the nanospheres were found to be superior to microspheres in regrowth of the optic nerve. The authors came to the conclusion that nanospheres’ intravitreal installation was more efficacious than microspheres due to delivering the therapeutics in the vitreous humor [55][56].
Giannavola et al., formulated acyclovir-loaded PLA nanosphere colloidal suspensions via a nanoprecipitation technique for ophthalmic drug delivery. They investigated the effect of molecular weight and type of polymer, as well as surfactants concentration used in the formulation on in vitro characteristics of nanosphere. The acyclovir-loaded nanospheres were tested in vivo for ocular drug absorption and the results were compared with a free-drug suspension. Moreover, PLA nanospheres were modified with the PEGylation technique to improve retention of formulation in the aqueous humor. The ocular tolerance test of PLA nanospheres was estimated by a modified Draize test. The drug concentration in the aqueous humor was monitored for 6 h to determined absorption and ocular bioavailability from different formulations. The higher molecular weight polymer led to reduced nanosphere size, whereas the PEGylated formulation showed sustained release, improved pharmacokinetics, and well toleration in the eye. The efficacy of PEGylated PLA nanospheres was reported significantly higher than plain PLA nanospheres [57].
11. Solid Lipid NPs (SLNPs)
This method is an emerging surrogate to colloidal drug delivery having the advantage of incorporating lipid and polymer NPs into a single system. A patent by Muller and Lucks disclosed SLNPs as stable solutions with a solid lipid core that have the potential for encapsulating medicaments stabilized by a layer of surfactant [58]. The SLNs differ from emulsions and liposomal systems in having high-melting lipids. They are free from chronic and acute toxicity, are biocompatible, and could provide a controlled and targeted release to a specific site. The lipid matrix plays an important role in controlling drug release and protecting the drug from degradable enzymes [59][60].
Fungal eye infection caused by fungal keratitis (FK) is a severe pathogenic condition that may lead to ocular morbidity. In this perspective, Natamycin (NAT)-loaded solid lipid NPs (NAT-SLNs), as first-line treatment of FK, has been designed to combat poor corneal permeation and improve residence time, bioavailability, and efficacy in the ocular tissue by Khames et al., The NAT-SLNs were developed by employing the emulsification–ultrasonication technique. The designed experiment was used to optimize the formulation, and the impacts of concentration of chosen factors, i.e., lipid, surfactant, and sonication time, were studied on particle size, surface charge, and drug encapsulation as responses. The optimized formulation was investigated in vitro for drug release, corneal permeation, and antifungal efficacy, as well as cytotoxicity studies. The outcomes of the optimized preparation reported a mean size of 42 nm and a surface charge of 26 mV, and drug entrapment was ~85%. NAT-SLNs expressed sustained drug release upto 10 h. In addition, NAT-SLNs improved corneal permeation, with a steady-state flux of 11.59 × 10−2 cm h−1 and permeability coefficient of 3.94 mol h−1, compared with plain drug, with a flux and permeability coefficient of 7.28 × 10−2 cm h−1 and 2.48 mol h−1, respectively. The antifungal activity revealed the enhanced zone of inhibition, 8 mm and 6 mm against Aspergillus fumigatus (ATCC 1022) and a clinical isolate of Candida albicans, respectively. The minimum inhibitory concentration (MIC) value was reduced to 2.5 times against each strain of fungus. Furthermore, the developed NAT-SLN formulation was nonirritant to the corneal tissue. The formulation NAT-SLNs resulted in extended drug release, enhanced corneal permeation, higher antifungal efficacy, and no toxic effects on the corneal tissues. Therefore, NAT-SLNs are among the anticipated ocular therapeutic systems for the treatment of corneal keratitis [61].
SLNPs have been widely explored as ocular nanocarrier systems to enhance drug absorption and improve the ocular bioavailability of both hydrophilic and lipophilic drugs. One study investigated the future of clarithromycin encapsulated SLNs in improving the permeation and penetration of drugs in topical ocular therapy. The formulation was developed using high-speed magnetic stirring, followed by the sonication technique. Solubility of the drug with different formulating components—namely, lipid former, surfactant (Tween 80), cosurfactant as transcutol P, and stearic acid was studied. The formulation of clarithromycin SLNs was optimized by statistical design and investigated in vitro for particle size, morphology, stability, ocular permeation, irritation, and pharmacokinetics studies. The drug from SLNs indicated ~80% release in 8 h and was complied with the Weibull kinetic release model. The optimized SLNs revealed a higher drug permeation of 30.45 μg/cm2/h, compared with the drug solution. The in vivo studies demonstrated that SLNs had 150% higher Cmax (~1066 ng/mL) and a 2.8-fold enhanced AUC (5736 ng h/mL), compared with the drug solution (Cmax; 655 ng/mL and AUC; 2067 ng h/mL). The result obtained concluded that SLNs serve as potential drug delivery carriers for enriching drug concentration in topical ocular delivery and could be efficacious in treating endophthalmitis [62].
Despite the application of statins in cardiovascular disease, it is largely explored in the management of age-related macular degeneration. To improve poor aqueous absorption in the ocular region, Yadav et al., investigated atorvastatin (ATS)-loaded SLNs as eye drops for self-use. They developed ATS-SLNs by hot, high-pressure homogenization and characterized in vitro in the ocular application. Their findings revealed that ATS-SLNs were 12-times more bioavailable in the ocular tissues than the conventional eye drop. The stability of the formulation was established as 13.62-times higher, including photostability. The fluorescein-labeled SLNs (F-SLNs) confirmed effective uptake of F-SLNs and prolonged ocular residence upto 7 h [63].
This entry is adapted from the peer-reviewed paper 10.3390/gels8020082
References
- Akhter, M.H.; Ahmad, A.; Ali, J.; Mohan, G. Formulation and Development of CoQ10-Loaded s-SNEDDS for Enhancement of Oral Bioavailability. J. Pharm. Innov. 2014, 9, 121–131.
- Nayak, K.; Misra, M. Triamcinolone Acetonide-Loaded PEGylated Microemulsion for the Posterior Segment of Eye. ACS Omega 2020, 5, 7928–7939.
- Kalam, M.A.; Alshamsan, A.; Aljuffali, I.A.; Mishra, A.K.; Sultana, Y. Delivery of gatifloxacin using microemulsion as vehicle: Formulation, evaluation, transcorneal permeation and aqueous humor drug determination. Drug Deliv. 2016, 23, 886–897.
- Perminaite, K.; Marksa, M.; Ivanauskas, L.; Ramanauskiene, K. Preparation of Ophthalmic Microemulsions Containing Lithuanian Royal Jelly and Their Biopharmaceutical Evaluation. Processes 2021, 9, 616.
- Junnuthula, V.; Boroujeni, A.S.; Cao, S.; Tavakoli, S.; Ridolfo, R.; Toropainen, E.; Ruponen, M.; van Hest, J.; Urtti, A. Intravitreal Polymeric Nanocarriers with Long Ocular Retention and Targeted Delivery to the Retina and Optic Nerve Head Region. Pharmaceutics 2021, 13, 445.
- Civiale, C.; Licciardi, M.; Cavallaro, G.; Giammona, G.; Mazzone, M.G. Polyhydroxyethyl aspartamide-based micelles for ocular drug delivery. Int. J. Pharm. 2009, 378, 177–186.
- Alami-Milani, M.; Zakeri-Milani, P.; Valizadeh, H.; Salehi, R.; Jelvehgari, M. Preparation and evaluation of PCL-PEG-PCL micelles as potential nanocarriers for ocular delivery of dexamethasone. Iran. J. Basic Med. Sci. 2017, 21, 153–164.
- Vaishya, R.D.; Gokulgandhi, M.; Patel, S.; Minocha, M.; Mitra, A.K. Novel Dexamethasone-Loaded Nanomicelles for the Intermediate and Posterior Segment Uveitis. AAPS PharmSciTech 2014, 15, 1238–1251.
- Mehra, N.; Aqil, M.; Sultana, Y. A grafted copolymer-based nanomicelles for topical ocular delivery of everolimus: Formulation, characterization, ex-vivo permeation, in-vitro ocular toxicity, and stability study. Eur. J. Pharm. Sci. 2021, 159, 105735.
- Patel, S.; Garapati, C.; Chowdhury, P.; Gupta, H.; Nesamony, J.; Nauli, S.; Boddu, S.H. Development and Evaluation of Dexamethasone Nanomicelles with Potential for Treating Posterior Uveitis After Topical Application. J. Ocul. Pharmacol. Ther. 2015, 31, 215–227.
- Xu, X.; Sun, L.; Zhou, L.; Cheng, Y.; Cao, F. Functional chitosan oligosaccharide nanomicelles for topical ocular drug delivery of dexamethasone. Carbohydr. Polym. 2019, 227, 115356.
- Diebold, Y.; Calonge, M. Applications of nanoparticles in ophthalmology. Prog. Retin. Eye Res. 2010, 29, 596–609.
- Kesavan, K.; Balasubramaniam, J.; Kant, S.; Singh, P.N.; Pandit, J.K. Newer approaches for optimal bioavailability of ocularly de-livered drugs: Review. Curr. Drug Deliv. 2011, 8, 172–193.
- Ahmad, M.Z.; Rizwanullah; Ahmad, J.; Alasmary, M.Y.; Akhter, H.; Abdel-Wahab, B.A.; Warsi, M.H.; Haque, A. Progress in nanomedicine-based drug delivery in designing of chitosan nanoparticles for cancer therapy. Int. J. Polym. Mater. 2021, 1–22.
- Bhatta, R.S.; Chandasana, H.; Chhonker, Y.S.; Rathi, C.; Kumar, D.; Mitra, K.; Shukla, P.K. Mucoadhesive nanoparticles for prolonged ocular delivery of natamycin: In vitro and pharmacokinetics studies. Int. J. Pharm. 2012, 432, 105–112.
- Musumeci, T.; Bucolo, C.; Carbone, C.; Pignatello, R.; Drago, F.; Puglisi, G. Polymeric nanoparticles augment the ocular hypotensive effect of melatonin in rabbits. Int. J. Pharm. 2013, 440, 135–140.
- Zhang, L.; Li, Y.; Zhang, C.; Wang, Y.; Song, C. Pharmacokinetics and tolerance study of intravitreal injection of dexame-thasone-loaded nanoparticles in rabbits. Int. J. Nanomed. 2009, 4, 175–183.
- Chi, H.; Gu, Y.; Xu, T.; Cao, F. Multifunctional organic–inorganic hybrid nanoparticles and nanosheets based on chitosan derivative and layered double hydroxide: Cellular uptake mechanism and application for topical ocular drug delivery. Int. J. Nanomed. 2017, 12, 1607–1620.
- Yu, A.; Shi, H.; Liu, H.; Bao, Z.; Dai, M.; Lin, D.; Lin, D.; Xu, X.; Li, X.; Wang, Y. Mucoadhesive dexamethasone-glycol chitosan nanoparticles for ophthalmic drug delivery. Int. J. Pharm. 2019, 575, 118943.
- Correa, Z.M.; Berry, J.L. Retinoblastoma. American Academy of Ophthalmology. 2016. Available online: https://www.aao.org/pediatric-center-detail/retinoblastoma-2016 (accessed on 16 December 2021).
- Bhavsar, D.; Subramanian, K.; Sethuraman, S.; Krishnan, U.M. Management of retinoblastoma: Opportunities and challenges. Drug Deliv. 2016, 23, 2488–2496.
- Akhter, M.H.; Beg, S.; Tarique, M.; Malik, A.; Afaq, S.; Choudhry, H.; Hosawi, S. Receptor-based targeting of engineered nanocarrier against solid tumors: Recent progress and challenges ahead. Biochim. Biophys. Acta BBA-Gen. Subj. 2021, 1865, 129777.
- Ahmad, J.; Ahmad, M.Z.; Akhter, M.H. Surface-Engineered Cancer Nanomedicine: Rational Design and Recent Progress. Curr. Pharm. Des. 2020, 26, 1181–1190.
- Akhter, M.H.; Ahsan, M.J.; Rahman, M.; Anwar, S.; Rizwanullah, M. Advancement in Nanotheranostics for Effective Skin Cancer Therapy: State of the Art. Curr. Nanomed. 2020, 10, 90–104.
- Akhter, M.H.; Amin, S. An Investigative Approach to Treatment Modalities for Squamous Cell Carcinoma of Skin. Curr. Drug Deliv. 2017, 14, 597–612.
- Akhter, M.H.; Rizwanullah, M.; Ahmad, J.; Amin, S.; Ahmad, M.Z.; Minhaj, A.; Mujtaba, A.; Ali, J. Molecular Targets and Nanopar-ticulate Systems Designed for the Improved Therapeutic Intervention in Glioblastoma Multiforme. Drug Res. 2021, 71, 122–137.
- Akhter, M.H.; Khalilullah, H.; Gupta, M.; Alfaleh, M.A.; Alhakamy, N.A.; Riadi, Y.; Shadab, M. Impact of Protein Corona on the Bi-ological Identity of Nanomedicine: Understanding the Fate of Nanomaterials in the Biological Milieu. Biomedicines 2021, 9, 1496.
- Qu, W.; Meng, B.; Yu, Y.; Wang, S. Folic acid-conjugated mesoporous silica nanoparticles for enhanced therapeutic efficacy of topotecan in retina cancers. Int. J. Nanomed. 2018, 13, 4379–4389.
- Kassem, M.; Rahman, A.A.; Ghorab, M.M.; Ahmed, M.; Khalil, R.M. Nanosuspension as an ophthalmic delivery system for certain glucocorticoid drugs. Int. J. Pharm. 2007, 340, 126–133.
- Yan, R.; Xu, L.; Wang, Q.; Wu, Z.; Zhang, H.; Gan, L. Cyclosporine A Nanosuspensions for Ophthalmic Delivery: A Comparative Study between Cationic Nanoparticles and Drug-Core Mucus Penetrating Nanoparticles. Mol. Pharm. 2021, 18, 4290–4298.
- Boddeda, B.; Boddu, P.; Avasarala, H.; Jayanti, V. Design and Ocular Tolerance of Flurbiprofen Loaded Nanosuspension. Pharm. Nanotechnol. 2015, 3, 56–67.
- Kausar, H.; Mujeeb, M.; Ahad, A.; Moolakkadath, T.; Aqil, M.; Ahmad, A.; Akhter, H. Optimization of ethosomes for topical thymoquinone delivery for the treatment of skin acne. J. Drug Deliv. Sci. Technol. 2019, 49, 177–187.
- Santos, G.A.D.; Ferreira-Nunes, R.; Dalmolin, L.F.; Ré, A.C.D.S.; Anjos, J.L.V.; Mendanha, S.A.; Aires, C.P.; Lopez, R.F.V.; Cunha-Filho, M.; Gelfuso, G.M.; et al. Besifloxacin liposomes with positively charged additives for an improved topical ocular delivery. Sci. Rep. 2020, 10, 19285.
- Lai, S.; Wei, Y.; Wu, Q.; Zhou, K.; Liu, T.; Zhang, Y.; Jiang, N.; Xiao, W.; Chen, J.; Liu, Q.; et al. Liposomes for effective drug delivery to the ocular posterior chamber. J. Nanobiotechnol. 2019, 17, 64.
- Shen, H.-H.; Chan, E.C.; Lee, J.H.; Bee, Y.-S.; Lin, T.-W.; Dusting, G.J.; Liu, G.S. Nanocarriers for treatment of ocular neovascularization in the back of the eye: New vehicles for ophthalmic drug delivery. Nanomedicine 2015, 10, 2093–2107.
- Natarajan, J.V.; Chattopadhyay, S.; Ang, M.; Darwitan, A.; Foo, S.; Zhen, M.; Koo, M.; Wong, T.T.; Venkatraman, S.S. Sustained release of an anti-glaucoma drug: Demonstration of efficacy of a liposomal formulation in the rabbit eye. PLoS ONE 2011, 6, e24513.
- Rajala, A.; Wang, Y.; Zhu, Y.; Ranjo-Bishop, M.; Ma, J.X.; Mao, C.; Rajala, R.V. Nanoparticle-assisted targeted delivery of eye-specific genes to eyes significantly improves the vision of blind mice in vivo. Nano Lett. 2014, 14, 5257–5263.
- Law, S.L.; Huang, K.J.; Chiang, C.H. Acyclovir-containing liposomes for potential ocular delivery. Corneal penetration and ab-sorption. J. Cont. Rel. 2000, 63, 135–140.
- Gupta, S.K.; Velpandian, T.; Dhingra, N.; Jaiswal, J. Intravitreal pharmacokinetics of plain and liposome-entrapped fluconazole in rabbit eyes. J. Ocul. Pharmacol. Ther. 2000, 16, 511–518.
- Zhang, R.; He, R.; Qian, J.; Guo, J.; Xue, K.; Yuan, Y.F. Treatment of experimental autoimmune uveoretinitis with intravitreal in-jection of tacrolimus (FK506) encapsulated in liposomes. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3575–3582.
- Keam, S.J.; Scott, L.J.; Curran, M.P. Verteporfin: A review of its use in the management of subfoveal choroidal neovascularisation. Drugs 2003, 63, 2521–2554.
- Vandamme, T.F.; Brobeck, L. Poly (amidoamine) dendrimers as ophthalmic vehicles for ocular delivery of pilocarpine nitrate and tropicamide. J. Control. Release 2005, 102, 23–38.
- Soiberman, U.; Kambhampati, S.P.; Wu, T.; Mishra, M.K.; Oh, Y.; Sharma, R.; Wang, J.; Al Towerki, A.E.; Yiu, S.; Stark, W.J.; et al. Subconjunctival injectable dendrimer-dexamethasone gel for the treatment of corneal inflammation. Biomaterials 2017, 125, 38–53.
- Lancina, M.G., III; Singh, S.; Kompella, U.B.; Husain, S.; Yang, H. Fast Dissolving Dendrimer Nanofiber Mats as Alternative to Eye Drops for More Efficient Antiglaucoma Drug Delivery. ACS Biomater. Sci. Eng. 2017, 3, 1861–1868.
- Torres-Luna, C.; Fan, X.; Domszy, R.; Hu, N.; Wang, N.S.; Yang, A. Hydrogel-based ocular drug delivery systems for hydrophobic drugs. Eur. J. Pharm. Sci. 2020, 154, 105503.
- Wang, P.; Chu, W.; Zhuo, X.; Zhang, Y.; Gou, J.; Ren, T.; He, H.; Yin, T.; Tang, X. Modified PLGA–PEG–PLGA thermosensitive hydrogels with suitable thermosensitivity and properties for use in a drug delivery system. J. Mater. Chem. B 2017, 5, 1551–1565.
- Chen, Y.; Li, Y.; Shen, W.; Li, K.; Yu, L.; Chen, Q.; Ding, J. Controlled release of liraglutide using thermogelling polymers in treatment of diabetes. Sci. Rep. 2016, 6, 31593.
- Phua, J.L.; Hou, A.; Lui, Y.S.; Bose, T.; Chandy, G.K.; Tong, L.; Venkatraman, S.; Huang, Y. Topical Delivery of Senicapoc Nanoliposomal Formulation for Ocular Surface Treatments. Int. J. Mol. Sci. 2018, 19, 2977.
- Sun, K.; Hu, K. Preparation and Characterization of Tacrolimus-Loaded SLNs in situ Gel for Ocular Drug Delivery for the Treatment of Immune Conjunctivitis. Drug Des. Dev. Ther. 2021, 15, 141–150.
- Noriaki, N.; Misa, M.; Saori, D.; Hiroko, O.; Hiroshi, S.; Naoki, Y. An in situ Gelling System Based on Methylcellulose and Tranilast Solid Nanoparticles Enhances Ocular Residence Time and Drug Absorption into the Cornea and Conjunctiva. Front Bioeng and Biotech. 2020, 8, 764.
- Akhter, M.H.; Rizwanullah; Ahmad, J.; Ahsan, M.J.; Mujtaba, A.; Amin, S. Nanocarriers in advanced drug targeting: Setting novel paradigm in cancer therapeutics. Artif. Cells Nanomed. Biotechnol. 2017, 46, 873–884.
- Katzer, T.; Chaves, P.; Bernardi, A.; Pohlmann, A.; Guterres, S.; Beck, R. Prednisolone-loaded nanocapsules as ocular drug delivery system: Development, in vitrodrug release and eye toxicity. J. Microencapsul. 2014, 31, 519–528.
- Rebibo, L.; Tam, C.; Sun, Y.; Shoshani, E.; Badihi, A.; Nassar, T.; Benita, S. Topical tacrolimus nanocapsules eye drops for therapeutic effect enhancement in both anterior and posterior ocular inflammation models. J. Cont. Rel. 2021, 333, 283–297.
- Li, F.; Hurley BLiu, Y.; Leonard, B.; Griffith, M. Controlled Release of Bevacizumab through Nanospheres for Extended Treatment of Age-Related Macular Degeneration. Open Ophthalmol. J. 2012, 6, 54–58.
- Robinson, R.; Viviano, S.R.; Criscione, J.M.; Williams, C.A.; Jun, L.; Tsai, J.C.; Lavik, E.B. Nanospheres delivering the EGFR TKI AG1478 promote optic nerve regeneration: The role of size for intraocular drug delivery. ACS Nano 2011, 5, 4392–4400.
- Akhter, M.H.; Madhav, N.S.; Ahmad, J. Epidermal growth factor receptor based active targeting: A paradigm shift towards advance tumor therapy. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1188–1198.
- Giannavola, C.; Bucolo, C.; Maltese, A.; Paolino, D.; Vandelli, M.A.; Puglisi, G.; Lee, V.H.L.; Fresta, M. Influence of Preparation Conditions on Acyclovir-Loaded Poly-d,l-Lactic Acid Nanospheres and Effect of PEG Coating on Ocular Drug Bioavailability. Pharm. Res. 2003, 20, 584–590.
- Müller, R.H.; Lucks, J.S. Arzneistoffträger Aus Festen Lipidteilchen-Feste Lipid Nanosphären (SLN). European Patent EP 0 605 497 B1, 20 March 1996.
- Badilli, U.; Gumustas, M.; Uslu, B.; Ozkan, S.A. Lipid-based nanoparticles for dermal drug delivery. In Organic Materials as Smart Nanocarriers for Drug Delivery; Grumezescu, A.M., Ed.; William Andrew Publishing: Bucharest, Romania, 2018; pp. 369–413.
- Ali, H.; Singh, S.K. Biological voyage of solid lipid nanoparticles: A proficient carrier in nanomedicine. Ther. Deliv. 2016, 7, 691–709.
- Khames, A.; Khaleel, M.; El-Badawy, M.; El-Nezhawy, A. Natamycin solid lipid nanoparticles—sustained ocular delivery system of higher corneal penetration against deep fungal keratitis: Preparation and optimization. Int. J. Nanomed. 2019, 14, 2515–2531.
- Nair, A.; Shah, J.; Al-Dhubiab, B.; Jacob, S.; Patel, S.; Venugopala, K.; Morsy, M.; Gupta, S.; Attimarad, M.; Sreeharsha, N.; et al. Clarithromycin Solid Lipid Nanoparticles for Topical Ocular Therapy: Optimization, Evaluation and In Vivo Studies. Pharmaceutics 2021, 13, 523.
- Yadav, M.; Schiavone, N.; Guzman-Aranguez, A.; Giansanti, F.; Papucci, L.; Perez De Lara, M.J.; Singh, M.; Kaur, I.P. Atorvastatin-loaded solid lipid nanoparticles as eye drops: Proposed treatment option for age-related macular degeneration (AMD). Drug Deliv. Transl. Res. 2020, 10, 919–944, Erratum in 2020, 10, 1531.
This entry is offline, you can click here to edit this entry!
