Salicylic acid (SA) promotes the formation axile roots and surface adventitious roots that originate from basal stem nodes of wheat grown under waterlogged conditions, but inhibits their elongation, leading to the formation of a shallow root system. The SA treatment also enhances the formation of axile roots in non-waterlogged plants but with only slight reductions in their length and branch root formation.
- salicylic acid
- waterlogging
- wheat
1. Introduction
Wheat (Triticum aestivum L.) is an important cereal crops worldwide; however, its production is adversely affected by several abiotic stress factors including waterlogging, which causes approximately 15–20% of the yield loss in annual wheat crop production [1]. Waterlogging significantly reduces the diffusion rate of oxygen through the soil, leading to the occurrence of hypoxic or anoxic conditions [2]. To cope with waterlogging-induced oxygen deficient conditions, plants develop several morphological and anatomical adaptive responses such as the formation of adventitious roots and root aerenchyma that improve oxygen availability to the roots [3]. Waterlogging induced development of adventitious roots in upland cereal crops such as wheat is often associated with the formation of aerenchyma [4,5].
Salicylic acid is one of the plant hormones that influences several plant physiological processes and mediates plant responses to a number of biotic and abiotic stresses [6,7]. With respect to abiotic stresses, SA plays an important role in mitigating the adverse effects of drought, cold, heat and salinity in many plant species including wheat [6,8]. Studies on Arabidopsis and mung bean have demonstrated the role of SA in the formation of adventitious roots under normal growing conditions [9,10,11]. For example, SA-deficient mutants of Arabidopsis eds5-1 and eds5-2 exhibit significantly reduced number of adventitious roots as compared to the corresponding wild type plants [11]. Further studies on mung bean revealed that induction of adventitious root formation by salicylic acid is closely associated with H2O2 accumulation. Consistently, treatment of mung bean hypocotyls with H2O2 scavenger/H2O2 biosynthesis inhibitor leads to inhibition of the SA-induced adventitious root formation [9,10]. In wheat, exogenous application of SA and its synthetic derivative, acetyl SA, has also been shown to have positive effects on several morphological traits in the shoot system including plant height, number of tillers, flag leaf area, number of spikes per plant, and number of grains per spike [12,13].
2. Salicylic Acid Enhances Adventitious Root and Aerenchyma Formation in Wheat under Waterlogged Conditions
Plant hormones such as ethylene and auxin play important roles in mediating plant adaptation to waterlogging/oxygen deficient conditions [4,14,15,16,17]. The observation of root morphological changes in response to waterlogging including enhanced formation of axile roots, and inhibition of axile root elongation and lateral roots emergence is consistent with previous reports [4,16,17, 18]. The association of these alterations in root morphological traits due to waterlogging with upregulation of specific root SA biosynthesis genes and induction of root SA content implies the significance of SA in forming a shallow root system and thereby tolerance to waterlogging conditions. However, as compared to the corresponding non-waterlogged plants, lower root SA level was evident in plants waterlogged for 1 and 7 days. Given that waterlogging induces accumulation of ethylene [17], which has been reported to suppress SA biosynthesis [19], the observed decrease in SA level might imply the responsiveness of SA biosynthesis to ethylene during the initial phases of waterlogging.
It has been shown previously that exogenous SA induces the development of adventitious roots in several plant species grown under normal or aerated conditions [9,10]. This effect of SA is reported to be associated with the activation of the de novo synthesis of auxin, which is known to play critical role in the formation of adventitious roots [20], and its rootward transport, and the repression of its shootward and non-polar transport in the root tips [21]. Consistent with these results, exogenous SA promoted the formation of axile roots in our non-waterlogged wheat plants. However, the induction of axile root formation in non-waterlogged plants was also prevalent in response to inhibition of SA synthesis, implying that the development of such roots is not entirely dependent on SA. The reduction of axile root length in the non-waterlogged wheat plants by SA along with its induction via inhibition of ethylene synthesis in the SA treated plants indicates the significance of ethylene in controlling the elongation of axile root cells. Consistently, ethylene has been shown to inhibit root growth in Arabidopsis plants grown under aerated condition through repressing cell elongation, which is mediated by its effect on local auxin biosynthesis and transport [22]. The observation of further induction in the formation of axile roots and reduction of root elongation in waterlogged plants due to exogenous SA indicates the importance of SA in promoting these root morphological traits and enhance adaptation to waterlogging. This result is in agreement with a previous report that showed the presence of higher SA level and development of more adventitious roots in waterlogging tolerant than waterlogging sensitive soybean lines grown under waterlogged condition [23]. Furthermore, exogenous SA has been shown to inhibit primary root elongation and lateral root formation but enhance adventitious root development, leading to the formation of a shallow root system in Arabidopsis [21]. The role of SA in suppressing primary root elongation and lateral root organogenesis has been reported to be associated with its negative effect on auxin transport through inhibiting protein phosphatase 2A, which regulates the activity of PIN auxin efflux carriers [24].
Despite inductions in the expression levels of stem node TaPAL5 and TaPAL6 genes and the level of SA in response to waterlogging for 7 days, no emergence of surface adventitious roots from the stem nodes was apparent at 14 DAWL. However, treatment of the plants waterlogged for 14 days with exogenous SA was able to promote the formation of these roots. Given that SA has been shown to induce adventitious root formation in a concentration dependent manner [9], our data suggest that the amount of SA produced by the basal stem nodes of the control/SA-untreated waterlogged plants was not sufficient to trigger this adaptive response. It has been shown previously that SA enhances adventitious root formation in Arabidopsis plants grown under aerated conditions through regulating the biosynthesis and transport of auxin [21], which has also been implicated in the regulation of re-differentiation of stem cells to form adventitious root primordia [20]. For instance, exogenous SA has been shown to induce the accumulation of free IAA and formation of adventitious roots in cucumber hypocotyls, and this induction of IAA accumulation by SA has been reported to be mediated by inhibition of IAA conjugation via competitive repression of the activity of auxin conjugating enzyme Gretchen Hagen3.5 [25]. On the other hand, treatment of cucumber hypocotyls with auxin transport inhibitor 1-naphthylphthalamic acid has been shown to completely inhibit SA-induced adventitious root formation. It is therefore likely that the exogenous SA applied to waterlogged wheat plants was able to induce auxin level and transport, and thereby trigger the formation of surface adventitious roots. In support of this, a close association between an increase in the level of auxin in the basal stem nodes and induction of surface adventitious root emergence has been observed in wheat plants waterlogged for durations longer than those considered in this study [4]. It is well established that ethylene is a key signaling molecule in promoting adventitious root formation in waterlogged plants including wheat [4,14,15,16]. The absence of any effect of inhibition of ethylene synthesis in non-waterlogged or waterlogged wheat plants treated with SA on SA-induced increases in the number of axile and/or surface adventitious roots indicate that SA regulates the formation of these roots independent of ethylene.
The prevalence of enhanced aerenchyma formation in the roots of both waterlogged and non-waterlogged wheat plants in response to exogenous SA, although its effect was more pronounced in waterlogged plants, indicates the role of SA in inducing this anatomical adaptive trait. Aerenchyma development, which facilitates oxygen transport and availability in waterlogged roots, is controlled mainly by ethylene [17,26]. Although the formation of aerenchyma in dry land plant species is uncommon under well-drained/aerated soil conditions, previous studies have reported that exogenous ethylene can promote the development of this anatomical trait in the roots of such plant species including wheat and maize when grown under aerated conditions [17,27]. Therefore, the repression of SA-induced aerenchyma formation in both non-waterlogged and waterlogged plants due to inhibition of ethylene synthesis suggests that the induction of aerenchyma development by SA is dependent at least partly on ethylene. In agreement with this, enhancement of aerenchyma formation in waterlogging tolerant as compared to waterlogging sensitive soybean lines grown under waterlogged condition has been shown to be associated with increases in the levels of both SA and ethylene but not that of ethylene alone [23].
The absence of any effect of exogenous SA on shoot growth under both non-waterlogged and waterlogged conditions suggests that SA does not have primary role in the regulation of shoot growth in wheat plants. In contrast, treatment with SA enhanced tiller formation under both conditions. The formation of tillers from axillary meristem is regulated by phytohormones such as cytokinin (CK) that acts as positive regulator of tillering, and auxin and strigolactones (SL), which act as inhibitors of tillering [28]. It has been shown previously that SA induces the expression levels of CK signaling genes such as those encoding the Arabidopsis histidine phosphotransfer (AHP6), which acts as positive regulator of CK signaling [29], and the Arabidopsis response regulator (ARR), which is a known mediator of CK signaling [30]. Furthermore, induction of tillering in tobacco plants overexpressing the Ras-related small GTP-binding protein 1 (RGP1) gene has been shown to be associated with increases in the levels of both CK and SA [31]. It therefore likely that the exogenous SA applied to our wheat plants induced CK production and signaling, and thereby tiller initiation. Previous reports have also implicated SA in the regulation of the biosynthesis, transport and signaling of auxin, which acts as important regulator of tiller formation/branching in plants. For example, SA represses the expression of genes encoding auxin receptor (TIR1) and auxin importer and exporter (AUX1 and PIN7) [32], and such suppression of auxin transport and signaling has been shown to lead to an increase in the number of tillers [33,34]. Therefore, the prevalence of enhanced tiller formation in response to SA might suggest the suppression of auxin production/signaling and its transport. Previous studies have also demonstrated the interaction between SA and SL. For instance, treatment of Arabidopsis seedlings with exogenous GR24, synthetic analogue of SL, induces SA level while SA level has been shown to be suppressed in more axillary growth2 (max2) mutant plants that are defective in SL signaling [35]. Therefore, the observation of induction in tiller formation in response to inhibition of SA biosynthesis in the control non-waterlogged wheat plants might imply suppression of SL signaling and thereby an increase in tiller number.
In summary, SA enhances the development of axile and surface adventitious roots in waterlogged wheat plants but represses their elongation, leading to the formation of shallow root system. This along with the pronounced induction of root aerenchyma formation by SA highlights the significance of SA in mediating morpho-anatomical responses and thereby adaptation of wheat plants to waterlogging as depicted in Figure 1.
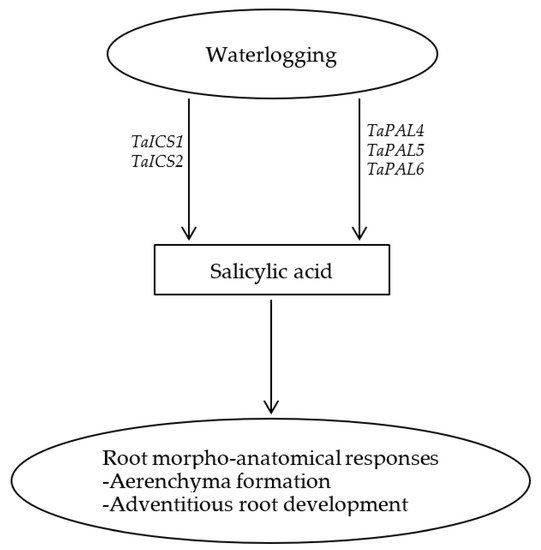
Figure 1. Schematic representation of the role of salicylic acid in inducing aerenchyma formation and development of adventitious roots in wheat under waterlogging condition.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23031243
References
- Herzog, M.; Striker, G.G.; Colmer, T.D.; Pedersen, O. Mechanisms of waterlogging tolerance in wheat—A review of root and shoot physiology. Plant Cell Environ. 2016, 39, 1068–1086.
- Armstrong, W. Aeration in higher plants. In Advances in Botanical Research; Woolhouse, H., Ed.; Academic Press: London, UK, 1980; pp. 225–332.
- Steffens, B.; Rasmussen, A. The physiology of adventitious roots. Plant Physiol. 2016, 170, 603–617.
- Nguyen, T.N.; Tuan, P.A.; Mukherjee, S.; Son, S.; Ayele, B.T. Hormonal regulation in adventitious roots and during their emergence under waterlogged conditions in wheat. J. Exp. Bot. 2018, 69, 4065–4082.
- Ploschuk, R.A.; Miralles, D.J.; Colmer, T.D.; Ploschuk, E.L.; Striker, G.G. Waterlogging of winter crops at early and late stages: Impacts on leaf physiology, growth and yield. Front. Plant Sci. 2018, 871, 1863.
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462.
- Zhao, P.; Lu, G.-H.; Yang, Y.-H. Salicylic acid signaling and its role in responses to stresses in plants. In Mechanism of Plant Hormone Signaling under Stress, II; Pandey, G.K., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 413–441.
- El-Shazoly, R.M.; Metwally, A.A.; Hamada, A.M. Salicylic acid or thiamin increases tolerance to boron toxicity stress in wheat. J. Plant Nutr. 2019, 42, 702–722.
- Yang, W.; Zhu, C.; Ma, X.; Li, G.; Gan, L.; Ng, D.; Xia, K. Hydrogen peroxide is a second messenger in the salicylic acid-triggered adventitious rooting process in mung bean seedlings. PLoS ONE 2013, 8, e84580.
- Kora, D.; Bhattacharjee, S. The interaction of reactive oxygen species and antioxidants at the metabolic interface in salicylic acid-induced adventitious root formation in mung bean [Vigna radiata (L.) R. Wilczek]. J. Plant Physiol. 2020, 248, 153152.
- Gutierrez, L.; Mongelard, G.; Floková, K.; Pǎcurar, D.I.; Novák, O.; Staswick, P.; Kowalczyk, M.; Pǎcurar, M.; Demailly, H.; Geiss, G.; et al. Auxin controls Arabidopsis adventitious root initiation by regulating jasmonic acid homeostasis. Plant Cell 2012, 24, 2515–2527.
- Matysiak, K.; Siatkowski, I.; Kierzek, R.; Kowalska, J.; Krawczyk, R. Effect of foliar applied acetylsalicilic acid on wheat (Triticum aestivum L.) under field conditions. Agronomy 2020, 10, 1918.
- Al-Rawi, O.H.; Abdukafoor, A.H.; Yousif, S.I.; Al-Shaheen, M. Effect of spraying with different levels of salicylic and humic acid in some growth characteristics and yield of wheat Triticum aestivum. Eurasia Proc. Sci. Technol. Eng. Math. (EPSTEM) 2018, 3, 133–138.
- Vidoz, M.L.; Loreti, E.; Mensuali, A.; Alpi, A.; Perata, P. Hormonal interplay during adventitious root formation in flooded tomato plants. Plant J. 2010, 63, 551–562.
- Dawood, T.; Yang, X.; Visser, E.J.W.; Te Beek, T.A.H.; Kensche, P.R.; Cristescu, S.M.; Lee, S.; Floková, K.; Nguyen, D.; Mariani, C.; et al. A co-opted hormonal cascade activates dormant adventitious root primordia upon flooding in Solanum dulcamara. Plant Physiol. 2016, 170, 2351–2364.
- Qi, X.; Li, Q.; Ma, X.; Qian, C.; Wang, H.; Ren, N.; Shen, C.; Huang, S.; Xu, X.; Xu, Q.; et al. Waterlogging-induced adventitious root formation in cucumber is regulated by ethylene and auxin through reactive oxygen species signalling. Plant Cell Environ. 2019, 42, 1458–1470.
- Yamauchi, T.; Watanabe, K.; Fukazawa, A.; Mori, H.; Abe, F.; Kawaguchi, K.; Oyanagi, A.; Nakazono, M. Ethylene and reactive oxygen species are involved in root aerenchyma formation and adaptation of wheat seedlings to oxygen-deficient conditions. J. Exp. Bot. 2014, 65, 261–273.
- Shiono, K.; Ejiri, M.; Shimizu, K.; Yamada, S. Improved waterlogging tolerance of barley (Hordeum vulgare) by pretreatment with ethephon. Plant Prod. Sci. 2019, 22, 285–295
- Li, Z.; Liu, H.; Ding, Z.; Yan, J.; Yu, H.; Pan, R.; Hu, J.; Guan, Y.; Hua, J. Low temperature enhances plant immunity via salicylic acid pathway genes that are repressed by ethylene. Plant Physiol. 2020, 182, 626–639.
- Guan, L.; Tayengwa, R.; Cheng, Z.M.; Peer, W.A.; Murphy, A.S.; Zhao, M. Auxin regulates adventitious root formation in tomato cuttings. BMC Plant Biol. 2019, 19, 435.
- Pasternak, T.; Groot, E.P.; Kazantsev, F.V.; Teale, W.; Omelyanchuk, N.; Kovrizhnykh, V.; Palme, K.; Mironova, V.V. Salicylic acid affects root meristem patterning via auxin distribution in a concentration-dependent manner. Plant Physiol. 2019, 180, 1725–1739.
- Růžička, K.; Ljung, K.; Vanneste, S.; Podhorská, R.; Beeckman, T.; Friml, J.; Benková, E. Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 2007, 19, 2197–2212.
- Kim, Y.H.; Hwang, S.J.; Waqas, M.; Khan, A.L.; Lee, J.H.; Lee, J.D.; Nguyen, H.T.; Lee, I.J. Comparative analysis of endogenous hormones level in two soybean (Glycine max L.) lines differing in waterlogging tolerance. Front. Plant Sci. 2015, 6, 714.
- Tan, S.; Abas, M.; Verstraeten, I.; Glanc, M.; Molnár, G.; Hajný, J.; Lasák, P.; Petřík, I.; Russinova, E.; Petrášek, J.; et al. Salicylic acid targets protein phosphatase 2A to attenuate growth in plants. Curr. Biol. 2020, 30, 381–395.e8.
- Dong, C.J.; Liu, X.Y.; Xie, L.L.; Wang, L.L.; Shang, Q.M. Salicylic acid regulates adventitious root formation via competitive inhibition of the auxin conjugation enzyme CsGH3.5 in cucumber hypocotyls. Planta 2020, 252, 75.
- Yamauchi, T.; Tanaka, A.; Mori, H.; Takamure, I.; Kato, K.; Nakazono, M. Ethylene-dependent aerenchyma formation in adventitious roots is regulated differently in rice and maize. Plant Cell Environ. 2016, 39, 2145–2157.
- Yamauchi, T.; Rajhi, I.; Nakazono, M. Lysigenous aerenchyma formation in maize root is confined to cortical cells by regulation of genes related to generation and scavenging of reactive oxygen species. Plant Signal Behav. 2011, 6, 759–761.
- McSteen, P. Hormonal regulation of branching in grasses. Plant Physiol. 2009, 149, 46–55.
- Zhang, X.; Dong, J.; Liu, H.; Wang, J.; Qi, Y.; Liang, Z. Transcriptome sequencing in response to salicylic acid in Salvia miltiorrhiza. PLoS ONE 2016, 11, e0147849.
- Badri, D.V.; Loyola-Vargas, V.M.; Du, J.; Stermitz, F.R.; Broeckling, C.D.; Iglesias-Andreu, L.; Vivanco, J.M. Transcriptome analysis of Arabidopsis roots treated with signaling compounds: A focus on signal transduction, metabolic regulation and secretion. New Phytol. 2008, 179, 209–223.
- Sano, H.; Seo, S.; Orudgev, E.; Youssefian, S.; Ishizuka, K. Expression of the gene for a small GTP binding protein in transgenic tobacco elevates endogenous cytokinin levels, abnormally induces salicylic acid in response to wounding, and increases resistance to tobacco mosaic virus infection. Proc. Natl. Acad. Sci. USA 1994, 91, 10556–10560.
- Wang, D.; Pajerowska-Mukhtar, K.; Culler, A.H.; Dong, X. Salicylic acid inhibits pathogen growth in plants through repression of the auxin signaling pathway. Curr. Biol. 2007, 17, 1784–1790.
- Jin, L.; Qin, Q.; Wang, Y.; Pu, Y.; Liu, L.; Wen, X.; Ji, S.; Wu, J.; Wei, C.; Ding, B.; et al. Rice dwarf virus P2 protein hijacks auxin signaling by directly targeting the rice OsIAA10 protein, enhancing viral infection and disease development. PLoS Pathog. 2016, 12, e1005847.
- Xu, K.; Sun, F.; Wang, Y.; Shi, L.; Liu, S.; Xi, Y. The PIN1 family gene PvPIN1 is involved in auxin-dependent root emergence and tillering in switchgrass. Genet. Mol. Biol. 2016, 39, 62–72.
- Rozpądek, P.; Domka, A.M.; Nosek, M.; Ważny, R.; Jędrzejczyk, R.J.; Wiciarz, M.; Turnau, K. The role of strigolactone in the cross-talk between Arabidopsis thaliana and the endophytic fungus Mucor sp. Front. Microbiol. 2018, 9, 441.
