This work was intended to enlarge the gates toward green organic technologies at room temperature, searching for new types of semiconductors with low toxicity and simple molecular organization. In our previous studies, para-aminobenzoic acid was used to construct a p-type green semiconductor. A non-toxic organic compound, acting as an electron donor, is sulpho-salicylic acid. SSA can be efficiently attached to the external shell of a ferrite (Fe3O4) nanocore, providing Fe3O4–SSA nanoparticles. This is a N-Type Organic Semiconductor - made by green technologies and used to construct a simple thin film transistor.
- Semiconductors
- Organic electronics
- Nano-core shell
1. Introduction
The latest advances in materials science successfully serve nanoelectronics’ interests, such as flexible electronic devices with elastomeric substrates [1], field-effect transistors attached to a gold electrode sensing pad for deoxyribonucleic acid hybridization [2], carbon-related materials such as diamond [3], or nanocomposites serving as efficient hole-transporting layers for organic solar cells [4]. Some organic materials present superior performance than inorganic materials for thin-film transistors (TFTs) [5]. A convenient method for the deposition of organic materials is dip-coating [6]. Polymers such as pentacene are the most widely used organic semiconductors for p-type materials nowadays [7], as well as for n-type materials under special conditions [8]. The precursors of pentacene are polycyclic aromatic hydrocarbons (PAHs), and their toxicity comes from the ability of these PAHs to bind to deoxyribonucleic acid inside cells [9]. Therefore, the green technologies are much sought after for solar cells [10] and other electronic devices [11]. OLED for display purposes was eco-friendly defined in terms of low power consumption and long lifetime [12]. However, after carrying out a search, zero results were returned for green technologies for n-type organic transistors, except one regarding green solvents [13].
This work was intended to enlarge the gates toward green organic technologies at room temperature, searching for new types of semiconductors with low toxicity and simple molecular organization. In our previous studies, para-aminobenzoic acid was used to construct a p-type green semiconductor [14]. A non-toxic organic compound, acting as an electron donor, is sulpho-salicylic acid (SSA), the chemical structure of which is presented in Figure 1. SSA can be efficiently attached to the external shell of a ferrite (Fe3O4) nanocore, providing Fe3O4–SSA nanoparticles using self-assembling techniques [15]. Essentially, an Fe3O4 nanocore represents an intrinsic semiconductor and SSA is suitable for organic electronic devices due to its molecular conjugation [16]. The self-assembly of SSA onto the external shell of ferrite nanoparticles easily occurs during the synthesis step, yielding core–shell nanoparticles with a good dispersibility in water. To create a demonstrator, we used a low-cost technology to deposit Fe3O4–SSA onto a compatible insulator on indium tin oxide (ITO)-coated glass. Finally, we tested the n-type characteristics of the Fe3O4–SSA film using a point-contact transistor. The Fe3O4–SSA film was contacted by two probes, i.e., the source and drain, and the ITO film was contacted by a third probe, i.e., the gate. This point-contact transistor, also named pseudo-MOS (Metal Oxide Semiconductor) or Ψ-MOSFET (Metal Oxide Semiconductor Field Effect Transistor) transistor, is specifically used for the in-situ electrical characterization of the conduction in thin semiconductors on insulators [17,18], including organic biomaterials [19,20].
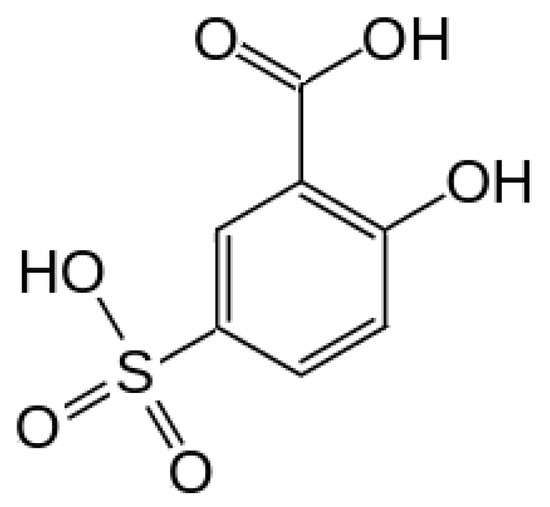
Figure 1. The chemical structure of sulpho-salicylic acid (SSA).
2. The n-Type Characteristics and the Low Toxicity of the Fe3O4–SSA Film
3. Conclusions
We demonstrated that an organic transistor with an Fe3O4–SSA film is operational. Obviously, many functional parameters have to be further optimized in the coming years to surpass the performance of the current OTFTs.
Herein, we investigated Fe3O4–SSA material as a candidate for green organic transistors. For this purpose, the synthesis of the Fe3O4–SSA material was based on co-precipitation. The FT-IR spectra confirmed the existence of SSA, while the TEM imaging captured the Fe3O4–SSA aggregates. The Fe3O4–SSA nanoparticles had good dispersion stability according to a zeta potential of +45.3 mV.
The point-contact OTFT transistor with an Fe3O4–SSA film presented an increasing drain current as the positive gate voltage increased, demonstrating the n-type character of the film. This was the main experimental argument for inducing an electron accumulation channel with a positive gate voltage. Compared to other OTFTs, our Fe3O4–SSA transistor presented a threshold voltage of approximately 5 V and an ION/IOFF ratio of 500, close to the parameters of a classical pentacene OTFT.
This entry is adapted from the peer-reviewed paper 10.3390/nano10091787
