1. Introduction
The fungi are a kingdom of eukaryotic heterotrophic organisms. As heterotrophic organisms, they obtain nourishment from the external environment by absorbing products through their walls
[1]. In addition to the well-known macroscopic fungi (such as mushrooms and molds), the kingdom includes many microscopic organisms such as yeasts and spores. Fungal cell walls typically are rigid and contain mainly chitin and glucans. Although some species are unicellular (e.g., yeasts), fungi are mainly multicellular and filamentous, with tubular morphology and a septate mycelium or without partitions. They give rise to modular forms with indeterminate growth. Fungal reproduction is complex. It has been estimated that a third of all fungi reproduce using more than one method of propagation. Fungi can reproduce asexually or sexually
[2]. The asexual reproduction occurs with the formation of special reproductive cells called spores, as result of mitosis in the parent cell (binary fission, budding and fragmentation); sexual reproduction occurs through the union of sex organs, cells or nuclei forming sexed spores, and the elements involved recombine their genetic information.
In the life cycle of a fungus, the anamorphic state during which the fungus reproduces asexually and the telomorphic state of sexual reproduction can be present, excluded or alternated. The most common way used to assign a fungus to a given group is based on the characteristics of sexual reproduction or using molecular data
[3]. Pathogenic insect species are few in the phyla Basidiomycota, Blastocladiomycota, Chytridiomycota and Kickxellomycotina, while a consistent number are recorded in Ascomycota and almost complete dominance occurs in Entomophthoromycota
[4].
Many common and important entomopathogenic fungi belong to the order Hypocreales of the Ascomycota
[5]. These include the asexual (anamorph) phases
Beauveria, Isaria, Hirsutella, Metarhizium, and the sexual (teleomorph) state
Cordyceps. The term entomopathogen refers to any member of the kingdom that can infect insects and other terrestrial arthropods, such as mites, ticks, and spiders. The heterotrophic metabolism forces them to a type of life dependent on a host and, depending on whether the relationship is neutral, of harm or advantage for the host organism, they are divided respectively into saprophytes, parasites, and symbionts. Researchers will mainly deal with parasitic entomopathogen fungi which are harmful to the host.
Numerous fungal microorganisms are pathogenic for many insect and nematode species and are able to control the natural populations of these by limiting their spread
[6]. There are more than one thousand species that infect and parasitize insects. Entomopathogenic Hypocreales are opportunistic pathogens highly adapted to infect insects and mites as a result of adaptations developed over time, such as the ability to overcome the host’s immune system defenses and the production of cuticular enzymes and degrading substances
[7]. They are globally and widely distributed in nature, ubiquitous across all environmental matrices, and can be easily grown in mass. Because they occur naturally, they can be considered generally environmentally friendly, with low to no residual toxicity to food and constitute a minimal concern for human safety
[8][9]. Consequently, they have been developed as microbial insecticides for the control of many important pest arthropods in agriculture, forestry and urban environments in different countries
[10].
2. Infection and Pathogenic Mechanisms
A suitable host becomes infected when it comes into contact with the spores. Properly, infection begins when the propagules adhere to the cuticle of a sensitive host
[11]. The adhesion of the spores to the epicuticle of the host, the subsequent germination and formation of the penetration structures (e.g., appressoria) are critical phases of the infection process
[11]. The penetration sites appear as dark and melanotic areas of the epicuticle
[12]. Entry requires the combined action of enzymatic degradation mechanisms and mechanical pressure. Many entomopathogens rely on the production of hydrophobic conidia to achieve rapid attachment to the waxy epicuticle.
The binding process is also promoted by molecules synthesized by the fungus called adhesins
[4]. This initial attack is immediately followed by the secretion of a mucus, with strong adhesive characteristics, and degradation enzymes
[13]. Entomopathogenic fungi produce a variety of cuticle-degrading enzymes
[14]. Proteases (among which are trypsin, chymotrypsin, esterase, collagenase and chymoelastase) are important for the penetration of
Metarhizium anisopliae and other fungi. Other enzymes, including endoproteases, esterases, lipases and chitinases, are involved
[15]. The action of the various enzymes can be supported by the secretion of organic acids. For example, the Basidiomycota are known to produce oxalic acid, while the genera
Rizophus and
Aspergillus produce fumaric, lactic, malic, citric and gluconic acids
[16]. Proteases (Pr1 and Pr2) and aminopeptidases are linked to the formation of appressoria
[17][18].
The appressorium is a specialized structure that represents a kind of narrow penetration peg useful for concentrating physical and chemical energy on a very small area of the cuticle
[19]. A successful attachment is followed by the growth of fungal hyphae within the cuticle interstices and the subsequent entry into the hemocoel. Within the hemocoel there is a transition from filamentous hyphal growth to the formation of small hyphal bodies with thin walls, similar to yeasts or protoplasts, which circulate in the hemolymph and proliferate. The advantages of this cellular form are the increase in nutrient acquisition rate and the ability to avoid the immune response system. Once the immune system is avoided, septicemia occurs. When the nutrients are depleted, the fungus returns to mycelial growth to invade the host’s internal tissues and organs (
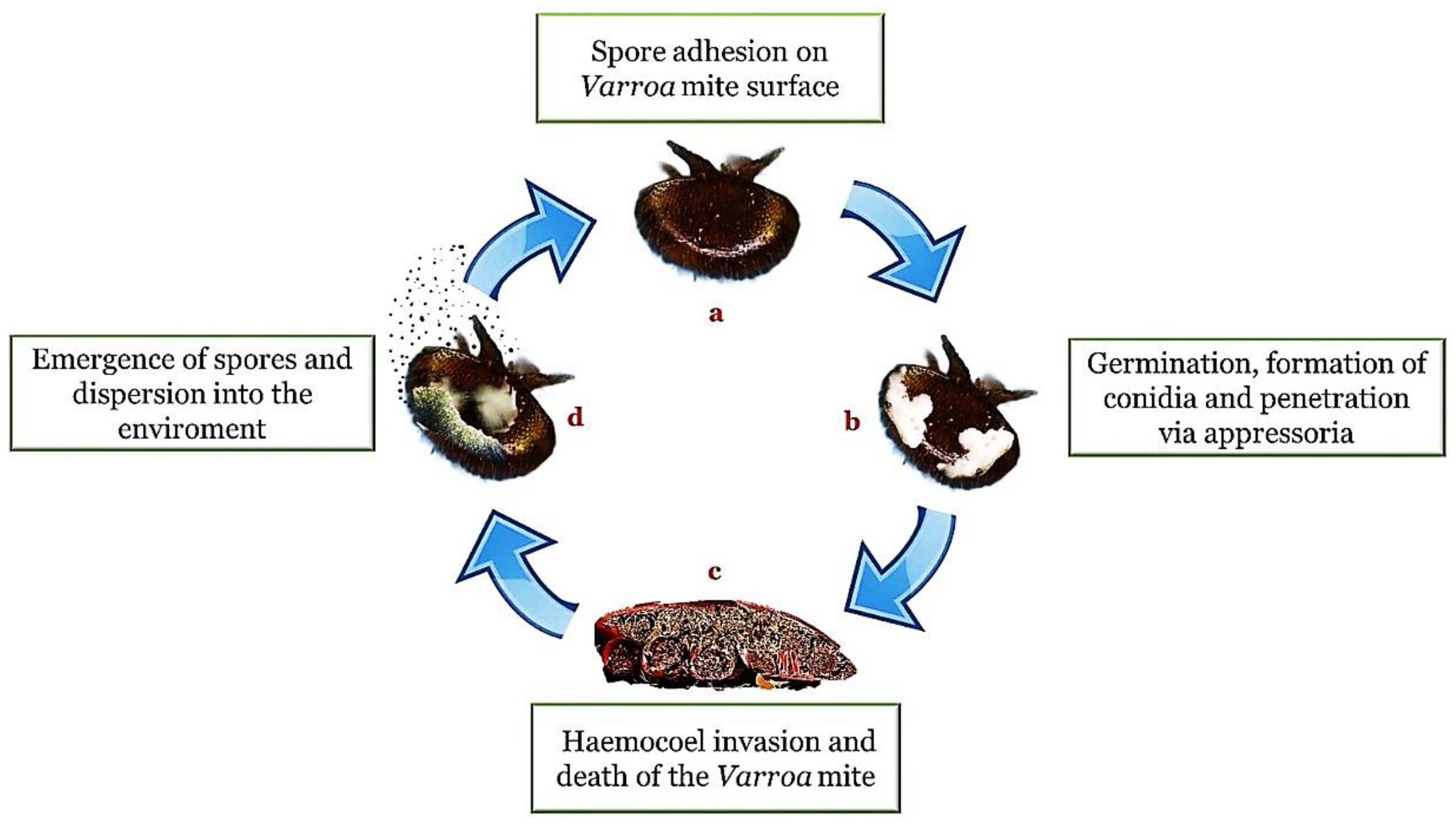
Figure 1).
Figure 1. Entomopathogenic infection cycle (method of action). In presence of a suitable substrate and a favorable environment, the adhesion of the spores (a) is followed by germination, the formation of hyphae and structures such as the conidia and the appressorium (b). Once penetrated inside the body cavity (haemocoel), the fungi cause a depletion of nutrients and a destruction of the tissues until the death of the host (c). The cycle is completed with emergence on the surface of the body for the propagation of the infectious elements in the external environment (d).
Insect death is the result of several factors, such as mechanical damage, nutrient depletion and the production of toxins in the insect’s body (toxicosis)
[20]. Several fungal toxins have been reported to be harmful to insect health. Paralysis, slowness, and reduced responsiveness to external stimuli in fungal-infected insects are symptoms attributable to the action of neuromuscular toxins. Toxins such as beauvericin, beauverolide, bassianolide and isarolide have been isolated from
B. bassiana [21].
Metarhizium spp. has been reported to produce different destruxins, with varying levels of activity and virulence, and cytochalasin
[22].
In addition to having an important role as suppressors of the immune response (prevention of nodulation and inhibition of phagocytosis), destruxins depolarize the muscle membrane of lepidopterans and influence the function of insect hemocytes
[23]. In general, the role of secondary metabolites is to facilitate the settlement of the pathogen in the host, causing paralysis and interrupting the physiological processes of the host and its immune responses. Due to the infection, the insect body is first damaged and then destroyed by the depletion of nutrients and the development of hyphae within it.
As a consequence of the progressive infection, the initially soft insect body stiffens due to the absorption of liquids by the fungus. The infection process is long and the length of time required for it to occur is approximately 6–14 days
[24]. After the death of the host and the exhaustion of nutrients, the hyphae of the fungus emerge from the corpse through the less sclerotic regions: holes of the body and intersegmental membrane. Spores are then produced on the outside of the corpse, partially or completely cover it and allow the conidia to spread and infect other individuals. Conidial dispersion is passive and relies primarily on wind, but other factors, such as rain, can also play a role in diffusion. Inside the hive, infected individuals of the population facilitate the mobilization of spores. After the conidia have been dispersed to another host, the cycle of infection begins again. The death of the insect can occur after a few days, with a variable time correlated to the fungus involved and the number of infecting spores.
3. Host Defense Mechanisms
Entomopathogens have developed a series of mechanisms aimed at establishing infection but, in a sort of coevolutionary arms race, the hosts have implemented a series of defense mechanisms useful to fight the infection. In addition to the limitations imposed by the environment, the fungal spores must overcome host defense mechanisms. The cuticular barrier and the peritrophic membrane covering the intestine represent two initial lines of defense
[25]. Although the epicuticle is an excellent substrate for fungal colonization, some of its constituents have been shown to possess antifungal properties. In particular, some secreted cuticular lipids represent an evolved defense mechanism useful for inhibiting adhesion and germination
[26]. Protease inhibitors are also produced at this level, inhibiting the enzymatic activity of the pathogens. During penetration, to reduce hemolymph leakage the hemostatic response is activated. This process involves proteins such as lipophorins, vitellogenin-like proteins, and calcium-dependent trans-glutaminases. The fungal penetration of cuticle is also followed by the activation of the prophenoloxidase cascade in epidermal cells, which will lead to the final synthesis of melanin. Melanin plays an important role in preventing the insect cuticle against fungal invasion. However, melanization is a line of defense against slow and weak growth of the pathogen but is not very effective against the more virulent strains. After a successful penetration into hemolymph, fungi propagate as yeast-like blastospores or hyphal bodies
[27]. Following this, the host’s cellular and humoral defense is activated. The cellular immune response relies on circulating hemocytes with the mechanisms of phagocytosis, nodulation, encapsulation, melanization, and production of protease inhibitors. The cellular response mechanism, which hemocyte cells put in place both when the cuticle is perforated by a foreign body and to eliminate targets too large to be phagocytosed (as in the case of parasites, protozoa, and fungi), is encapsulation. The mechanisms of the functioning of the insect immune system have been studied mainly in
Drosophila and, subsequently, have been applied to other genera
[28]. In particular, there are three differentiated hemocyte types: plasmatocytes, which make up most of the cells in circulation and act as phagocytes of bacteria; crystal cells, involved in the melanization reaction and that produce prophenoloxidase; lamellocytes, which mediate the immune encapsulation reaction to large infectious objects that are too large for phagocytes to ingest
[29][30].
Massive production of hemocytes in the hematopoietic organs is induced within hours of infestation, resulting in an increase in the number of lamellocytes. These begin to build a series of cell layers around the non-self, forming the hemocyte capsule. They lose their cytoplasmic content, which includes the phenoloxidase that allows melanization to start. A key enzyme in melanin synthesis is phenoloxidase (PO), synthesized in the form of zymogen (prophenyloxydase or PPO) by different types of hemolymph cells, such as crystal cells and lamellocytes
[31]. The recognition of pathogens and/or lesions leads to the activation of a series of serine proteases, with consequent proteolytic cleavage of the inactive PPO and formation of the active PO, which catalyzes the formation of phenolic and quinone compounds
[32].
Once formed, melanin is deposited around invading microorganisms
[32]. Inside the capsule, the non-self is killed by the production of cytotoxic radicals, such as reactive oxygen species (ROS) and reactive nitrogen species (RNS), or by asphyxiation
[33][34]. Quinonic substances and ROS are produced as intermediates during the synthesis of melanin and have a cytotoxic action. The invasion of the hemocoel triggers the humoral immune response system, which leads to the production of a series of host antifungal immune effectors. The systemic immune response takes longer to establish than the cellular response
[35]. It represents the last line of immune defense against persistent infections
[36].
Antimicrobial peptides (AMPs) are the main effectors of the humoral immune response. AMPs are peptides, generally cationic, which in the hemolymph can reach very high concentrations
[37]. The main production site of these molecules is the fat body
[38]. Several types of antimicrobial peptides have been identified including defensins, cecoprins, diptericins, apidaecins and hymenoptaecins. The defensins have a broad spectrum of action: they have cytotoxic activity against Gram-positive and some Gram-negative bacteria, fungi and protists
[39]. The trigger of the immune response is in fact activated by the recognition of the intruder by receptors such as Pattern Recognition Receptors (or PRRs) and ꞵ-glucan-binding proteins, which recognize molecular motifs associated with pathogens (Pathogen Associated Molecular Patterns), such as lipolysaccharides (LPS), peptidoglycans (PGN) and beta-1.3 glycans
[40]. The humoral immune response against a fungal invasion is mediated by the Toll pathway.
Recognition of fungal microorganisms by the Toll-like receptor (TLR) triggers a cascade reaction which, involving signaling intermediates, leads to the translocation of transcription factors from the cytoplasm to the nucleus. This process results in the production of antimicrobial peptides (AMP) in the fat body and their subsequent secretion within the hemolymph. The Toll pathway has receptors capable of recognizing PGNs that have L-lysine residues
[41].
The other two major pathways activated by fungal infection are the JNK and the JAK/STAT pathways, both of which are involved in the synthesis of antibacterial AMP and stress/injury response proteins. Other important immune-related molecules produced by insects are lysozime, apolipophorin III, hemocyanin and transferrin. The expression of transferrin is increased during B. bassiana and M. anisopliae infection suggesting its important role in the infection.
In addition to cellular and humoral immunity, social insects show a higher level of defense which finds its expression in grooming behavior. The grooming behavior and behavioral changes in infected insects, such as behavioral fever exhibited by infected grasshoppers
[42], may help the host against fungal colonization and the development of the disease. Another example is the hygienic behavior exhibited by the honey bee in the presence of
Ascophaera apis infection: the workers identify larvae infected and remove them. From another point of view, the grooming behavior may be harmful. The infectious propagules removed with mouthparts are stored in the infrabuccal cavity within the buccal chamber and infection can occur if inocula germinate. However, it has been hypothesized that chitinase secretions within the oral cavity have a fungistatic activity.
Although not yet fully understood, entomopathogenic fungi have developed evolutionary strategies and adaptations that allow them to evade or overcome their host’s antifungal cell defense system. Among them is the assumption of a morphological change once inside the hemocoel. For Hypocreales, this involves the transition from hyphal growth to the formation of small thin-walled hyphal bodies, similar to the hyphal bodies of yeast (also known as blastospores). This form speeds up the dispersion through the hemolymph and avoids the detection by pathogen recognition molecules, although they are still susceptible to plasmatocyte phagocytosis
[43]. More virulent fungal strains are also capable of overcoming the hemocyte response causing a reduction in the number of granulocytes
[44][45]. Many of the entomopathogenic fungi in the Entomophthoromycota phylum take the form of protoplasts (similar to blastospores), which helps them evade detection by hemocytes
[46].
Furthermore, entomopathogenic fungi mask the immunogenic carbohydrates of the surface to avoid immune stimulation and secrete secondary metabolites that cause paralysis, interference with the physiological processes of the host and, mainly, suppression of the immune responses.