Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Microbiology
Diabetic foot ulcers (DFU) are a growing concern worldwide as they pose complications in routine clinical practices such as diagnosis and management. Bacterial interactions on the skin surface are vital to the pathophysiology of DFU and may control delayed wound healing.
- microbiota
- dysbiosis
- probiotics
- diabetes
- wound healing
1. On the Horizon: Gut-Skin Microbiome Axis
Multiple research findings revealed that the gut microbiome performs a prominent role in several skin disorders. Not only is the skin microbiome altered, but also surprisingly many skin diseases are accompanied by an altered gut microbiome [21]. Several reports described the gut-skin axis, which shows the relationship between the gut microbiota and the skin. It is one of the most promising research areas currently targeting the microbiome of the skin, which plays a critical role in controlling the cutaneous processes critical to human health and disease [40]. Additionally, the overall constitution of various microbial communities observed on the skin primarily depends on the physiology of the different sites of our skin, with alteration in the relative abundance of bacterial taxa associated with moist, dry, and sebaceous microenvironments. It is profound that some of the lipophilic Propionibacterium species mainly influenced the sebaceous sites. Microbes that prefer moist, humid environments, such as Staphylococcus and Corynebacterium species, were more prevalent in areas that had a high moisture content, such as elbow bends and feet [41].
A skin microbiome is a group of complex communities that contain different species of microbes. It is a highly dynamic microbial community that helps maintain an interdependent relationship with the host [42]. In addition, the human gut is microbially inherited, and the skin barrier shares striking common features with gut microbes. The countless purposes and functions of gut and skin microbes are incredibly similar, as discovered through research on the microbiome over the past few years. Both are highly innervated and vascularized. They are essential for various immune and neuroendocrine functions and are therefore important for the assembly of the gut–skin axis [42].
The gastrointestinal tract’s inner surface and the outer surface of skin cells are layered by epithelial cells (ECs) that are directly connected to the exogenous environment. The epithelial cells help to maintain an important connection between both the internal body and the external environment; thus, they act as first line of defense, preventing various microbes from entering the gastrointestinal tract [43]. There is evidence to support the existence of a bi-directional association between the skin and the gut that also combines the possible connection between gastrointestinal health and skin homeostasis and allostasis in humans [44]. Moreover, the immune system is continuously primed to differentiate between harmful and beneficial compounds in our bodies; according to some reports, there is a profound bidirectional relationship [45].
Microbes inherited in our gastrointestinal tract play an important role in our daily lives; the gut and skin have so many similar characteristics. These highly vascularized, well-perfused, and intensively innervated structures are colonized with distinguishable microbial populations and represent as vital contact organ systems by which mammalian organisms communicate with their surroundings. Further to that, these relatively small populations of microbes are complex immune and neuro-endocrine organs that perform an important role in the immune and endocrine systems of the entire body. As a consequence, both from an evolutionary and medical standpoint, their proper functioning is critical for maintaining homeostasis and survival. The intestinal microbiota is the ‘virtual organ,’ with substantial immunological and metabolic implications. It has an effect on the several organ systems, including skin. Other studies have also suggested its involvement in skin health primarily due to modifying the immune system in humans [46,47,48].
The concept of the “skin-gut axis” has surfaced in past few years and has been an important scientific platform; nevertheless, pathobiological understandings are all still suffering from a lack. Although the pathways clarifying how well the gut and skin interact really are not precise, it is likely to be involved a complex interrelationship between the nervous, immune, and endocrine systems, as well as environmental factors [42,49]. A gut-skin axis communicates mostly through metabolites, the neuroendocrine system, diet, and the central nervous system, according to research hypotheses. Nonetheless, the gut-skin correlation is primarily determined by modifications in gut microbiota and their products, as well as indirectly by changes in the gut epithelium’s diet, which influences the intestinal flora and the skin [50]. The close interconnection between the gut and skin is beyond doubt. Perhaps, both the intestinal microbes themselves and their metabolic by-products govern the skin physiology.
The theories underpinning how well the gut-skin axis functions are still being explored, though there are some concepts that have been recommended: (1) bacterial products as well as diet might very well modify the physiology of the intestinal epithelium, causing numerous secretory products which may circulate systemically to reach the skin; (2) neurotransmitters, hormones, and other biologically active chemicals derived from intestinal flora, including SCFAs, could function on receptor sites within the skin and it may effectively change the skin or alter the skin’s commensal microbes; and (3) ingested substances and chemicals, at first when absorbed, might indeed change the skin or alter the function [50].
5. Microbiota Involved in Wound Healing
Normal wound healing takes place as a basic biological system inside the human body through four precise and highly programmed stages, hemostasis, inflammation, proliferation, and remodeling. For any wound to recover fully, only those four phases should happen, and in the right order and time scale. Normal skin wounds heal in approximately one to two months. It is indeed a natural, biological, and sophisticated process that occurs after a tissue injury and tends to involve blood cells, connective tissue, parenchymal cells, ECM, as well as soluble mediators such as cytokines and growth factors communicating each other during the wound healing mechanism [82]. Microbial colonization occurs in any and all types of wounds e.g., acute well as chronic, and there is a break in epithelial barrier which characterizes a wound impairs the factors that influence and constrain the microbial community at that site. A wound is be associated with the physical interruption in the integrity of the epithelium as well as the subsequent host immune response to fix this break. Any breach in the epithelial barrier impedes the events that shaped and confine the intestinal microbiota at around that site. Destabilization of the epithelium diminishes mucus or lipid production, distorts anti-microbial peptide representation and stimulates inflammatory cascades. Because mucosal surfaces are exposed to the environment, wounds allow non-indigenous microbes to colonize the site while also changing the forces that stabilize indigenous microbial colonization [83]. Over the last few years, considerable evidence on the human microbiome already reinforced the hypothesis that the ecological microbial community in/on humans is crucial in the host for maintaining the homeostasis, and also any internally or externally factors that cause dysbiosis of the skin commensal microbiome in diabetes patients also may prompt the shambles of immunologic stabilization inside the skin and therefore would empower the onset of varying skin diseases [41]. Furthermore, under various physiological conditions in a healthy individual, the observed mechanism of cutaneous epidermal repair is highly efficient; however, when this process stalls due to various external and internal factors in the host, the function of tissue deteriorates to reclaim structural and functional integrity, resulting in the formation of chronic wounds [84]. It is preferable to stabilize signaling factors, which include growth for effective wound healing; however, down-regulation of such factors contributes to the pathophysiology of DFUs. Additional factors known to promote wound healing delay in diabetes involve macro- and microvascular, neuropathic, immune function, and microbiome disturbances [85].
Much previous research investigated the implicated mechanism associated with wound healing, and recognized that the whole framework is, generally speaking, not straightforward. It involves an interaction between multiple cell types, primarily epidermal keratinocytes, neutrophils, and macrophages, and the inherited resident commensal microbiota of the gastrointestinal tract in humans. Wound colonization was observed in the later mechanism, accelerating wound healing by influencing the host’s innate immune system. Keratinocytes have expanded and migrated as part of the mechanism, and fibroblasts also exited and amassed the extracellular matrix (ECM) during this stage of wound healing. During the proliferation phase, angiogenesis takes place. During the remodeling phase of this entire mechanism, the extracellular matrix (ECM) reconstructs the appearance of scar formation, followed by the recovery of the epidermal skin barrier. The epithelial barrier will not fully heal when one of the phases is interrupted in just about any way, leading to the formation of a chronic wound. Furthermore, wounds provide a desirable moment of opportunity for microbiota to acquire access to the underlying tissues in order to colonize and grow further [86,87].
Scientists also expanded on the ongoing relationship between cutaneous and gastrointestinal microbes, claiming that any changes in local cutaneous and gastrointestinal microflora may positively or negatively impact wound healing via various pathways. One of these is that it primarily affects the host through the production of antimicrobial molecules and the regulation of the host’s inflammatory and immune response [88,89,90,91]. Furthermore, clinical assessment clearly shows that impeded wound healing is a strong predictor of mortality and morbidity in a considerable number of people with diabetes worldwide [92].
Microbes can also have an adverse effect on the wound healing process. Specific bacteria, such as Staphylococcus aureus, have been linked to wound infections and complications. More specifically, known microbes such as Staphylococcus, Anaerococcus, Corynebacterium, Porphyromonas, and Streptococcus are abundant in the chronic wound microbiota. [93,94]. In addition to cutaneous microflora, intestinal microflora influences wound healing by directly or indirectly attempting to influence a variety of healing factors including tissue oxygenation levels, blood pressure, inflammation, and the immune system [93]. Despite the high oxygen levels in chronic wounds, anaerobes such as Fingelodia, Prevotella, Peptonipihlus, Peptostreptococcus, and Anaerococcus have emerged as major threats [95].
6. Altered Microbiota in Diabetic Wound
Amputations of lower limbs due to diabetic foot ulcers accounted for 40–70% of all non-traumatic amputations, according to research findings. Foot ulcers occur prior in approximately 85 percent of all amputations in diabetics. When the ulcer progresses to its most complicated form, treatment becomes more difficult; in many cases, diabetic patients must be admitted to hospital [96]. Surprisingly, one of the findings looked at a significant difference in the abundance of microbial communities in the healthy skin of the foot and forearm of 30 diabetic patients versus 30 healthy people. The results revealed a statistically significant change in the microbial community as well as skin diversity in diabetic forearms but not in non-diabetic’s forearms. The phylum Firmicutes is more prevalent in non-diabetic foot skin, whereas Actinobacteria, specifically the species Corynebacterium, is more abundant in diabetic foot skin and has been linked to higher Staphylococcus aureus carriage rates [97].
Bacterial interactions on the skin’s surface play a critical role in the pathophysiology of diabetic foot ulcers (DFU). It is essential in the wound healing mechanism and may contribute to healing delays when there are numerous unfavorable conditions [98]. The host-microbe interface is frequently cited as a critical point in the development of wound infections. The clinical judgment, however, concluded that the observed number of pathogenic microbial species at this interface is lower when compared to the presence of many commensal bacteria. Furthermore, many of the species found in chronic wounds are commensals in healthy skin, and there are clear differences in the composition and diversity of the microbiota in diabetic foot ulcers (DFU) and healthy skin microbiota [99].
It is critical to place the recent acknowledgment of the microbiome’s impacts on health in an evolutionary context. With the advancement of microbiome research, various groups of scientists identified a possible link between altered microbiota and various diseases. However, it remains a mystery whether such changes are the cause or the result of various diseases, or whether various diseases cause an altered microbiota composition. The microbial composition of human skin is not always static; the presence and abundance of different types of microbes in skin wounds are primarily determined by the type of wound observed. However, it is known that the three major phyla identified in pressure ulcers, namely Firmicutes, Proteobacteria, and Actinobacteria are very similar to those found in healthy commensals [100].
Preclinical research is increasingly demonstrating compelling evidence and agreement that microorganisms in the gut influence many beneficial functions in humans. Furthermore, Ammons et al. conducted research on the presence of microbes in diabetic patients [100] and studies have expanded its concept such that, while the diversity of bacteria was independent of chronic wound type, there were more prevalent bacteria such as S. epidermidis identified in patients with diabetic foot ulcers, and Pseudomonas aeruginosa exhibited with a higher relative abundance overall in patients with chronic wounds demonstrating biofilm formation [101].
Many other studies were conducted to determine the types of microbes found in DFU. In general, three to five species of microorganisms are identified in an infected DFU, which consists primarily of Gram-positive aerobes (Staphylococcus aureus, Staphylococcus epidermidis, Corynebacterium spp.); Gram-positive anaerobes (Enterococcus spp., Propionibacterium spp., Streptococcus spp., Peptostreptococcus spp., Peptococcus spp.); Gram-negative aerobic microbes (Pseudomonas aeruginosa, Acinetobacter spp.); Gram-negative anaerobes (Proteus mirabilis, Escherichia coli, Bacteroides spp.); and fungi including the Candida spp. [102,103]. It also depicted a higher prevalence of Gram-negative pathogens in low-income countries; the most common bacteria observed in DFU is Pseudomonas aeruginosa [104,105].
A 16SrDNA pyrosequencing was performed by Wolcott et al. [32]. There were 910 patients with chronic diabetic foot ulcers among the total of 2963 patients. The results of this study show that diabetic patients’ chronic wounds had a significantly higher prevalence of Staphylococcus species. Furthermore, there was a high abundance of Pseudomonas species in the chronic wound samples, which included a variety of other species such as P. aeruginosa. However, Corynebacterium—a traditional commensal that made up more than 1% of the total bacterial population in more than one-third of the samples. Despite the fact that chronic cutaneous wounds are subjected to relatively high levels of oxygenation, a large number of anaerobic bacteria were found in the wound samples. Finegoldia spp. were found in 25% of the wounds, while Prevotella spp., Peptoniphilus spp., and Anaerococcus spp. were found in 12, 16, and 18% of the wounds respectively [32].
Multiple independent, culture-based studies found that Gram-positive cocci (GPC) are the most consistently isolated microbes from DFU patients. Furthermore, Staphylococcus aureus is the most commonly observed species, accounting for more than 50% of all wounds, followed by coagulase-negative Staphylococci spp. and Streptococcus spp. [106,107,108,109]. More evidence suggests that Staphylococcus spp. and Corynebacterium spp. are common in wounds, followed by a plethora of diverse anaerobic communities in DFU patients [110,111]. In the study conducted by Kalan and colleagues, the most abundant genera investigated in descending order were Staphylococcus (18.95%), Corynebacterium (14.64%), Pseudomonas (9.37%), and Streptococcus (7.32%) [111].
The Shotgun metagenomics analysis from the DFU patients showed S. aureus as the major Staphylococcus species and was dominated by a single strain, S. aureus 7372, from Staphylococcal species present in lesser abundance included the coagulase-negative species such as S. pettenkoferi, S. epidermidis, S. simulans, and S. lugdunensis. Corynebacterium striatum, a bacterium that has been associated with infection and multi-drug resistance [112], was the most prevalent Corynebacterium spp. classified in DFU and showed a positive correlation with ulcer duration, while C. jeikeium, C. amycolatum, C. pseudogenitalium, C. tuberculostearicum, and C. resistens were present in lesser abundances. Pseudomonas spp. were the third most abundant genera detected, with the most abundant species identified as P. aeruginosa followed by P. alcaliphila. P. aeruginosa that is a commonly known pathogen associated with DFU as it is frequently isolated by culture-based methods. Streptococcus was the fourth most abundant genera, with S. agalactiae, S. dysgalactiae, and S. anginosus present in patients with DFU [111]. Many experiments have been conducted in recent years by various groups of scientists to better understand the role of microbiota in the wounds of DFU patients. According to some studies, when biofilm occurs in DFU patients, the most abundant components observed are various species of Staphylococcus as well as some diverse anaerobes; some groups of scientists also reported the presence of Pseudomonas aeruginosa as prevalent in DFU patients. We summarized the observed microbes in the gut, skin, wounds, and DFU mentioned in the manuscript, in Table 1. Based on existing knowledge of wound microbiota in DFU patients, the higher or lower abundance of microbes such as various strains of Staphylococcus spp. with some other anaerobes mentioned above may enable clinicians and scientists to make a thorough diagnosis of individual wounds, which may lead to improved patient prognoses through the selection of optimal treatment strategies that could be used in hospitals. Figure 2 describes the altered microbiota in diabetic wound healing [111,113] and Figure 3 describes the observed microbiota involved in skin, wounds, and DFU.
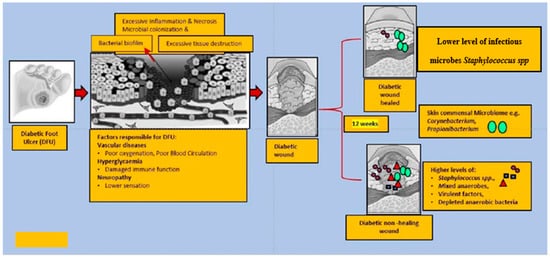
Figure 2. This figure explains the altered microbiota in diabetic wound healing. In general, diabetic foot wounds are complicated by various factors contributing to impaired tissue regeneration. Several factors impairing wound healing and associated factors are hyperglycaemia, peripheral neuropathy, vascular disease, and a complex microbiome. It is challenging to identify microbial communities that assemble in wound tissue and have not necessarily been associated with cardinal signs of infection. A debridement elicited reduced diversity of bacteria, governed by decreased anaerobic bacterial abundance in the overall community. One subset of wounds achieved complete re-epithelialization within 12 weeks. Kalan et al. [111] investigated the role of colonizing microbiota in wound healing, clinical outcomes, and a response to therapy in patients with chronic diabetic wounds. Strains of the wound pathogen S. aureus were associated with poor outcomes, and sharp debridement therapy depleted anaerobic bacteria in wounds with favorable outcomes.
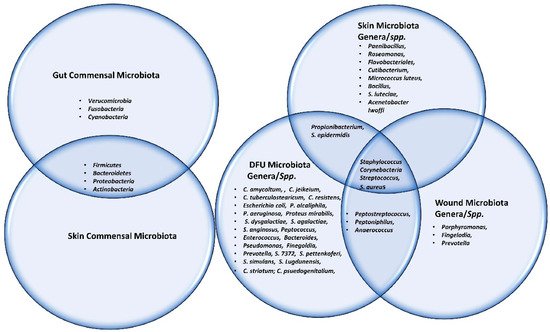
Figure 3. This Venn diagram depicts all the microbes (commensal, genera as well as spp.) present in gut, skin, wound and DFU.
7. Probiotics Therapy in Diabetic Wound Healing
Because probiotics are live microorganisms, they are non-pathogenic bacterial strains that have many beneficial effects, such as improvement of normal gastrointestinal microbiota in the host, particularly when consumed in required proportions [24]. Increased evidence that is associated with the use of probiotics in wound healing and infection in various diseases, including DFU, has emerged in recent years [24].
Choundappan and group [114] showed that local administration of probiotics, specifically Lactobacillus Plantarum (5 billion CFU) strain, improved wound healing in 36 DFU patients. In this experiment, the probiotics solution was applied to the wound at the time of dressing every day. The wound swab culture was examined on several occasions, including day 0, day 5, and day 10. The findings of this study were promising, with the number of wounds with a positive status decreasing as the course progressed in either group of patients. At the end of day 5, eight individuals in the intervention group had negative wound swab cultures, while only six individuals in the control group had negative wound swab cultures. On day 10, 12 subjects in the intervention group had negative wound cultures, whereas 10 in the control group had positive wound cultures. This study concluded that probiotics can be used safely in the treatment of infected diabetic wounds by hastening the wound healing process, as shown by a significant difference in the wound bed score on day 7 [114].
Another clinical trial using the probiotics on DFU patients was performed by Mohseni et al. [115]. The study included a randomized double-blind, placebo-controlled trial of probiotics supplementation in 60 DFU patients. The patients were divided randomly into two different groups to obtain everyday either a probiotics capsule that consisted of Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus Fermentum, and Bifidobacterium bifidum (2 × 109 CFU/g each) or a placebo (n = 30) for a period of 12 weeks. The outcome of the study was promising and it showed significant beneficial effects specifically on the size of ulcer, the level of glycemic control, the cholesterol, plasma nitric oxide, the total antioxidant capacity that supports the diabetes wound healing mechanism.
Mohtashami et al. [116] published a study that demonstrated the use of probiotics and their beneficial effects in the diabetes wound healing mechanism. It was performed on Wistar rats, and it claimed that the animals’ wound healing process was accelerated when compared to untreated wounds. Lactobacillus bulgaricus and Lactobacillus plantarum bacteria strains were used as probiotics in the treatment of diabetes wounds. The duration of diabetic wound healing was 94–98% approximately in 14 days observed in the probiotic treatment group, which is consistent with the duration of wound healing observed in other studies.
Campos et al. also conducted a study to assess the impact of perioperative probiotic administration on the cutaneous healing process in diabetic rats. The rats in this study were given the Probiatop and were given probiotics (P) orally [117]. According to the study conducted by Mohseni et al., the probiotic is primarily composed of a mixture of four different strains of bacteria such as Lactobacillus paracasei LPC-37, Bifidobacterium lac-tis HN0019, Lactobacillus rhamnosus HN001, and Lactobacillus acidophilus NCFM at doses of 1 × 109 CFU/g [115]. The experimental design consisted of two distinct groups—groups were given a probiotic mixture or maltodextrin for a continuous five days prior to the creation of the skin excisional wound. Consumption was continued until the day of euthanasia. The promising result revealed that peri-operative probiotic supplementation in diabetic rats promotes improved skin healing, attenuation of the inflammatory response, accelerated wound neovascularization, increased wound type I collagen deposition, and weight loss prevention. Glycemic control in the animals was shown to be improved. Lactobacilli’s beneficial effect was tested on a mouse model, where Lactobacilli bacteria were transformed into CXCL12-producing vectors to bioengineer the wound microenvironment after topical application. Lactobacillus reuteri, which expressed CXCL12, stimulated immune cells. The healing process is propelled by immune cells [118]. Overall, the frequent communication within the gut–brain–skin axis may represent a strong link between the gut microbiome and cutaneous health. However, these connections, as well as the exact mechanism involved, are still poorly understood. Probiotics may provide a potentially beneficial therapeutic approach that can safely alter the gut–skin axis and modify systemic health in patients with wound healing disorders. Furthermore, it is necessary to comprehend the interaction between the host’s respective pathways and the beneficial microbiota. It would also be beneficial to describe in detail the therapeutic potential of topical probiotics and how beneficial bacteria could alter the gut-skin axis in modifying systemic health in patients suffering from various disorders. Given the increased research on probiotics and the important role they play in human health, their use as an integrative treatment opens up a new avenue for treating patients with wound healing disorders [119]. The review published by Wang et al. [120] summarizes the possible link between gut microbial flora, probiotics, and diabetes, concentrating on the procedure through which probiotics relieved diabetes explicitly by targeting intestinal microbiota from different aspects of oxidative stress, immune responses, amino acid metabolism, intestinal permeability, and short-chain fatty acids (SCFA). Overall, the findings of the study have laid the groundwork for future clinical research and development efforts to identify a possible group of microbes with anti-diabetes effects that can be used as probiotics to improve intestinal homeostasis and alleviate metabolic diseases such as diabetes. These effects, however, have only been determined for Lactobacillus microbial species at this time. Further research is needed to investigate the range of effective bacterial strains that can be used as probiotics to lower glucose levels in diabetes and could be another factor to consider in the prevention of chronic wounds and thus facilitate faster wound healing in DFU [121]. However, the causal relationship between an imbalance in intestinal flora and diabetes, as well as the underlying mechanism(s), has not been fully established; further clinical trials in DFU patients are required.
It is not uncommon for external or internal factors to alter the balance between the skin and skin microbiome causing skin disorders, infection, and impaired wound healing. A wound’s microbes and pathogens are exposed to a broad range of microenvironments during wound formation and healing. As wounds heal, microenvironments increasingly change. Therefore, microbes respond physiologically to enhance the host’s innate immune system or to prevent pathogenic infection from the primary or opportunistic pathogens. Stress suppresses the production and localization of AMP, impairs barrier permeability, and increases susceptibility to infection; researchers have already published evidence to support this conclusion. It is possible that [122,123] may delay wound healing, including DFU. Based on the description given in this review, supplementation with beneficial microbes, e.g., probiotics, during stressful times or in the cases of skin dysbiosis may promote wound healing.
In this review, we discussed how probiotics, both orally and topically delivered, influence wound healing in DFU. Probiotics are known to aid in wound healing by stimulating the production of immune cells, and they also have antagonistic effects against pathogens via competitive exclusion of pathogens [124]. According to a recent publication [27], the skin and gut have different morphologies but share some physiological characteristics. Interactions between the gut and skin are centered on microbiota and metabolites secreted by them, which can interfere with biological processes regulating metabolism, immunity, inflammation, oxidative stress, and neuroendocrine function. The mechanism of action by which gut health affects skin health (from the inside out) is critical for defining cross-communication between the two compartments. The finding presented an essential aspect of gut microbiota, skin homeostasis, and skin wound healing with probiotics during the gut-skin axis.This discovery revealed an important aspect of gut microbiota, skin homeostasis, and skin wound healing with probiotics within the gut-skin axis. Based on the aforementioned studies, this review also supports the role of the gut-skin axis in wound healing in DFU. Therefore, based on the currently available literature, well-designed clinical trials, systematic review results, and various experimental findings, it would be ideal for clinical doctors and researchers to focus on clinical trials specifically targeting DFU patients to investigate the influence of probiotics on wound healing. It also necessitates understanding the role of beneficial bacterial strains in wound healing mechanisms, identifying the strain, determining the optimal dose, and determining the duration of perioperative supplementation. As a result, the use of these bacterial strain mixtures found in probiotics can be regarded as a challenging therapeutic approach for the treatment of diabetic wounds.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23042375
This entry is offline, you can click here to edit this entry!
