Asthma is a common chronic respiratory inflammatory disease. It is characterized by airway hyperresponsiveness, allergen-specific IgE secretion, mucus hypersecretion, and airway inflammation. In recent years, the incidence of asthma has increased due to increases in indoor dust, pollen, toxic particles, environmental pollutants, and other allergens. It has been reported that many traditional Chinese herbs are effective in reducing asthma symptoms in both humans and animals. Eupatilin, a pharmacologically active flavone extracted from Artemisia argyi, has a variety of pharmacological activities, including anti-inflammatory, anticancer, antioxidant, antiallergic, cardioprotective, and neuroprotective activities. In the present study, eupatilin was found to attenuate OVA-induced asthma by modulating NF-κB, MAPK and Nrf2 signaling pathways for the first time. Eupatilin may be a promising therapeutic agent for the treatment of asthma.
1. Effect of Eupatilin on Inflammatory Cells in the Bronchoalveolar Lavage Fluid (BALF)
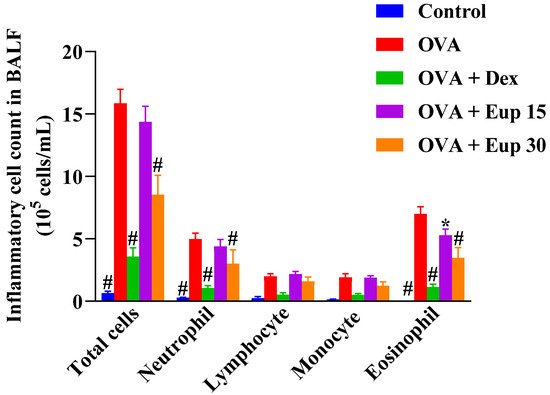
The effect of eupatilin on the profile of inflammatory cells in the BALF was detected. As shown in Figure 2, compared with the control group, the OVA group showed significantly increased numbers of total inflammatory cells, neutrophils, lymphocytes, monocytes, and eosinophils in the BALF. Eupatilin administration could reduce the number of inflammatory cells in the BALF, especially neutrophils and eosinophils, to varying degrees, indicating that eupatilin could alleviate inflammatory cell infiltration in the lungs of asthmatic mice.
Figure 2. Effect of eupatilin on OVA-induced inflammatory cell count in the BALF. Twenty-four hours after the last challenge, the total inflammatory cells, neutrophils, lymphocytes, monocytes, and eosinophils in the BALF were counted. Data represent the mean ± SEM (n = 7). * p < 0.05, # p < 0.0001 vs. OVA group.
2. Eupatilin Reduces OVA-Induced Th2 Cytokine Levels in the BALF and OVA-IgE Levels in the Serum
In asthma, inflammatory responses are closely associated with the activation of Th2 cells [
28]. Th2 cytokines can activate eosinophils and induce B cells to produce IgE [
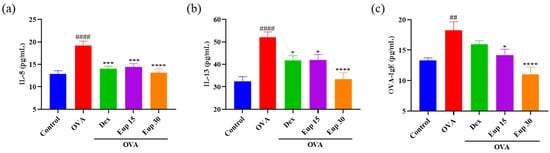
29]. Compared with control treatment, OVA sensitization and challenge significantly increased the production of IL-5, IL-13, and OVA-IgE (
Figure 3). However, eupatilin administration inhibited the increase of these cytokines in a dose-dependent manner, and it was also significantly more effective than dexamethasone at a dose of 30 mg/kg.
Figure 3. Effect of eupatilin on OVA-induced Th2 cytokines in the BALF and OVA-IgE levels in the serum were detected by ELISA. (a) BALF IL-5 levels. (b) BALF IL-13 levels. (c) Serum OVA-IgE levels. Data represent the mean ± SEM (n = 7). ## p < 0.001, #### p < 0.0001 vs. control group; * p < 0.05, *** p < 0.001, **** p < 0.0001 vs. OVA group.
2. Effect of Eupatilin on Lung Histological Changes in Asthmatic Mice
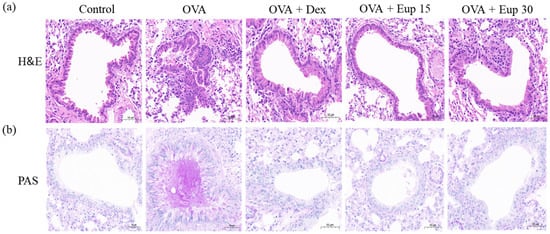
Inflammatory cell infiltration and mucus secretion are the key features of allergic asthma. Hematoxylin and eosin (H&E) and periodic acid-Schiff (PAS) staining analyses were used to detect histological changes in lung tissues. As shown in Figure 4a, lung tissues from the OVA group exhibited significant inflammatory cell infiltration. Additionally, PAS staining showed that the mice in the OVA group overproduced mucus (Figure 4b). However, eupatilin treatment could significantly improve the inflammatory cell infiltration induced by OVA and remarkably inhibit mucus hypersecretion, which were comparable with the effect of dexamethasone.
Figure 4. Effect of eupatilin on lung histological changes in OVA-induced asthmatic mice. (a) H&E staining was used to detect inflammatory cell infiltration. (b) PAS staining was used to detect the production of mucus around the airways. 200× magnification; scale bar: 50 μm.
4. Effect of Eupatilin on NF-κB, MAPK and Nrf2 Signaling Pathways in Asthmatic Mice
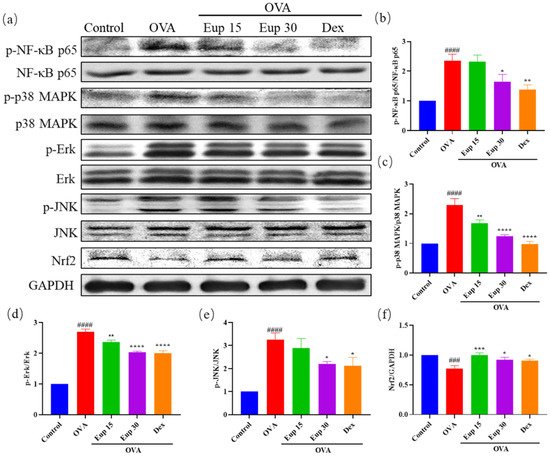
NF-κB and MAPK are key targets associated with inflammation and play an important role in asthma inflammation. To explore the effect of eupatilin on NF-κB and MAPK signaling pathways, the protein levels of p-NF-κB p65, p-p38 MAPK, p-Erk, and p-JNK were detected by Western blotting (Figure 5a). Compared with OVA induction alone, eupatilin treatment significantly inhibited the OVA-induced phosphorylation of NF-κB (Figure 5b). Furthermore, we observed that the levels of phosphorylated MAPK family members (p38 MAPK, Erk, and JNK) were significantly enhanced in the OVA group, whereas eupatilin treatment significantly inhibited the activation of p38 MAPK, Erk, and JNK (Figure 5c–e).
Figure 5. Effect of eupatilin on NF-κB, MAPK, and Nrf2 signaling pathways in OVA-induced asthmatic mice. (a) Western blotting analyses of NF-κB p65, p-NF-κB p65, p38 MAPK, p-p38 MAPK, Erk, p-Erk, JNK, p-JNK, and Nrf2 protein expression in lung tissues. (b) Quantification of the p-NF-κB p65/NF-κB p65 ratio. (c) Quantification of the p-p38 MAPK/p38 MAPK ratio. (d) Quantification of the p-Erk/Erk ratio. (e) Quantification of the p-JNK/JNK ratio. (f) Quantification of the Nrf2/GAPDH ratio. Data represent the mean ± SEM (n = 3). ### p < 0.001, #### p < 0.0001 vs. control group; * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 vs. OVA group.
Inflammation itself leads to oxidative stress in the airways and lungs, further exacerbating the inflammatory response [
30]. Therefore, the protein expression levels of Nrf2 in lung tissues were evaluated. The results show that OVA administration significantly inhibited Nrf2 expression, while Nrf2 expression levels were significantly increased after eupatilin administration, and the effect was comparable with that of dexamethasone (
Figure 5f). These results suggest that eupatilin could alleviate the inflammatory reactions in asthma by affecting NF-κB, MAPK, and Nrf2 signaling pathways.
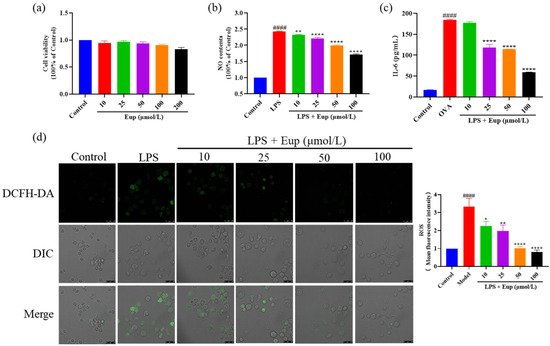
5. Effect of Eupatilin on Inflammatory Cytokines in RAW264.7 Cells
After treatment with different concentrations of eupatilin, the observed cell viability was not significantly different from that of the control group, indicating that eupatilin had no significant cytotoxicity up to a dose of 200 μmol/L (Figure 6a). Compared with the LPS group, eupatilin significantly reduced the release of NO and IL-6, with eupatilin acting in a dose-dependent manner (Figure 6b,c). Furthermore, LPS significantly induced ROS production in RAW264.7 cells, while eupatilin significantly inhibited ROS production (Figure 6d). These results suggest that eupatilin had anti-inflammatory activity in LPS-stimulated RAW264.7 cells.
Figure 6. Effect of eupatilin on inflammatory cytokines in LPS-stimulated RAW264.7 cells. RAW264.7 cells were induced with 1 µg/mL LPS and treated with various concentrations (10, 25, 50, and 100 µmol/L) of eupatilin for 24 h. (a) Cell viability after treatment with different concentrations of eupatilin for 24 h. (b) NO levels in cell supernatants after LPS and eupatilin treatment. (c) IL-6 levels in cell supernatants after LPS and eupatilin treatment. (d) ROS levels in RAW264.7 cells treated with LPS and eupatilin. 630× magnification; scale bar: 25 μm. Data represent the mean ± SEM (n = 4). #### p < 0.0001 vs. control group; * p < 0.05, ** p < 0.01, **** p < 0.0001 vs. LPS group.
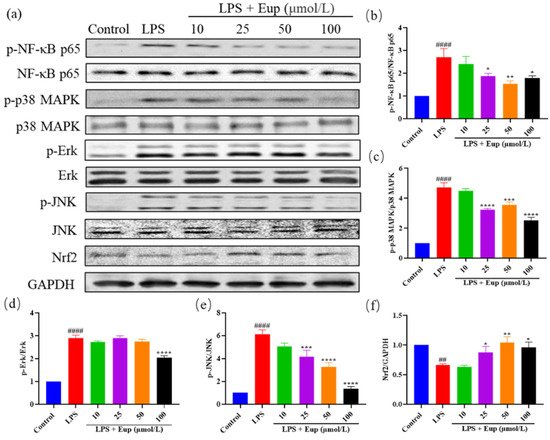
6. Effect of Eupatilin on NF-κB, MAPK, and Nrf2 Signaling Pathways in RAW264.7 Cells
To further verify the therapeutic target of eupatilin in asthma, we detected the protein expression of p-NF-κB p65, p-MAPK, and Nrf2 in LPS-stimulated RAW264.7 cells (Figure 7a). The levels of phosphorylated NF-κB p65, p38 MAPK, Erk, and JNK in the LPS group were significantly increased, while eupatilin significantly inhibited the phosphorylation of these proteins (Figure 7b–e). In addition, compared with the LPS group, the eupatilin group showed significantly elevated expression of Nrf2 (Figure 7f). The protein expression trends in LPS-stimulated RAW264.7 cells were consistent with those in mouse lung tissues, further indicating that eupatilin exerted its anti-inflammatory effects in asthma through effects on NF-κB, MAPK, and Nrf2 signaling pathways.
Figure 7. Effect of eupatilin on NF-κB, MAPK, and Nrf2 signaling pathways in LPS-stimulated RAW264.7 cells. After RAW264.7 cells were treated with LPS and eupatilin for 24 h, intracellular proteins were extracted for subsequent Western blotting analysis. (a) Western blotting analyses of NF-κB p65, p-NF-κB p65, p38 MAPK, p-p38 MAPK, Erk, p-Erk, JNK, p-JNK, and Nrf2 protein expression in LPS-stimulated RAW264.7 cells. (b) Quantification of the p-NF-κB p65/NF-κB p65 ratio. (c) Quantification of the p-p38 MAPK/p38 MAPK ratio. (d) Quantification of the p-Erk/Erk ratio. (e) Quantification of the p-JNK/JNK ratio. (f) Quantification of the Nrf2/GAPDH ratio. Data represent the mean ± SEM (n = 3). ## p < 0.01, #### p < 0.0001 vs. control group; * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 vs. LPS group.
7. Conclusion
Asthma is a recurrent chronic airway inflammatory disease involving multiple inflammatory cells and mediators [
32,
33]. Glucocorticoids are considered to be the best choice to treat asthma. However, they have limited efficacy and can cause various adverse reactions. Therefore, there is an urgent need to find a new, safer, and more effective drug to treat asthma [
34,
35]. Pharmacological and phytochemical studies have identified many potential anti-inflammatory ingredients, especially options derived from traditional Chinese medicine, so traditional Chinese herbal medicine is becoming an important source of active drugs [
36]. Eupatilin has good anti-inflammatory activity, but its therapeutic activity in asthma has not been explored. Our results confirm that eupatilin attenuated OVA-induced asthma by inhibiting NF-κB and MAPK and activating Nrf2 signaling pathways.
The inflammatory responses in asthma involve the excessive production of IgE by B cells, release of inflammatory cytokines, and infiltration of inflammatory cells [
37]. A variety of inflammatory cells are involved in airway inflammation, such as macrophages, eosinophils, lymphocytes, and neutrophils [
38,
39]. Among them, eosinophils are the main contributors to allergic inflammation and are involved in the induction of airway hyperresponsiveness and remodeling in asthma [
40,
41].
In OVA-induced asthmatic mice, eupatilin reduced the numbers of inflammatory cells, especially neutrophils and eosinophils. Eupatilin also decreased the levels of IL-5, IL-13 in the BALF and OVA-IgE in the serum. In addition, eupatilin treatment could significantly improve inflammatory cell infiltration induced by OVA and could remarkably inhibit mucus hypersecretion. Furthermore, eupatilin inhibited the activation of NF-κB and MAPK pathways and increased the expression of Nrf2 in OVA-induced asthmatic mice. In vitro, eupatilin significantly reduced LPS-stimulated NO, IL-6, and ROS production. Additionally, the NF-κB, MAPK, and Nrf2 protein expression in LPS-stimulated RAW264.7 cells was consistent with that in OVA-induced asthmatic lung tissues.
In summary, eupatilin attenuated OVA-induced asthma by regulating NF-κB, MAPK, and Nrf2 signaling pathways. Eupatilin may be a promising therapeutic agent for the treatment of asthma.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23031582