Osteoporosis is a chronic debilitating disease caused by imbalanced bone remodeling processes that impair the structural integrity of bone. Over the last ten years, the association between fibroblast growth factor 23 (FGF23) and osteoporosis has been studied in both pre-clinical and clinical investigations. FGF23 is a bone-derived endocrine factor that regulates mineral homeostasis via the fibroblast growth factor receptors (FGFRs)/αKlotho complex. These receptors are expressed in kidney and the parathyroid gland. Preclinical studies have supported the link between the local actions of FGF23 on the bone remodeling processes. In addition, clinical evidence regarding the effects of FGF23 on bone mass and fragility fractures suggest potential diagnostic and prognostic applications of FGF23 in clinical contexts, particularly in elderly and patients with chronic kidney disease. However, inconsistent findings exist and there are areas of uncertainty requiring exploration.
1. Introduction
Osteoporosis is a chronic debilitating disease caused by an imbalance in bone remodeling processes that favor bone resorption over bone formation. Structural integrity of the bone is maintained through intricate and interrelated activities of bone-forming osteoblasts, bone-resorbing osteoclasts, physicochemical conditions that modulate local matrix mineralization, and systemic mineral homeostasis. Over the last ten years, the association between fibroblast growth factor 23 (FGF23) and osteoporosis has been studied [
1,
2,
3,
4]. FGF23 is a bone-derived endocrine factor that regulates phosphate and vitamin D homeostasis via the fibroblast growth factor receptors (FGFRs)/αKlotho complex. These receptors are expressed in kidney and the parathyroid gland [
5,
6,
7]. The FGF23-mediated mechanism interacts with the classical calcium/phosphate regulating processes driven by parathyroid hormone (PTH) and calcitriol (active vitamin D). In addition to the systemic effects of FGF23, preclinical studies have revealed mechanistic insights in the local actions of FGF23 on bone remodeling processes [
8,
9]. Moreover, accumulating evidence from clinical studies reported the association between FGF23, bone remodeling and fragility fracture in the elderly, either with or without a decline in renal function. These recent advances in the insights regarding FGF23 effects in mineral homeostasis and bone remodeling suggest potential clinical applications of FGF23 in the clinical context. However, inconsistent findings exist and there are areas of uncertainty requiring an exploration.
2. Role of FGF23 in Postmenopausal and Age-Related Osteoporosis Pathogenesis
Osteoporosis is primarily caused by an imbalance in the remodelling process. In the early stages of postmenopausal osteoporosis, a deficiency of estrogen induces an increase in RANKL expression, which results in increased osteoclast numbers and activity as well as concurrent suppression of osteoblast functions. Subsequently, increased net bone resorption outpacing bone formation caused rapid loss of mainly trabecular bone mass. As previously discussed, FGF23 inhibits bone mineralization by suppressing TNAP and resulting in PPi accumulation in both physiological and supraphysiological conditions. Thus, increased FGF23 could accentuate the net negative remodelling by inhibiting bone formation. Although some studies found that physiological concentration of increased FGF23 was associated with increased osteoblast differentiation and bone nodule formation, these might not be direct actions of FGF23 on osteoblasts but could be a compensatory response to the inhibition of bone formation. The auto-/paracrine inhibitory effect of FGF23 is also confirmed that indicated that excessive levels of FGF23 decrease ALP activity in differentiated osteoblasts, whereas the absence of FGF23 signalling results in a Klotho-independent, cell-autonomous increase in ALP activity. Moreover, FGF23 may regulate the bone remodelling balance by inhibiting the early stages of osteoclastogenesis from osteoclast progenitors and promoting osteoclast-mediated bone resorption.
In the second longer phase (age-related osteoporosis), the impaired bone quality is the gradual loss of mineral from bones with aging, which are also influenced by other age-related conditions, such as deconditioning or frailty, vitamin D deficiency, and secondary hyperparathyroidism. An imbalance of systemic mineral homeostasis in the elderly particularly impaired renal function, hyperphosphatemia, and additionally led to an increase in FGF23 secretion, resulting in a progressive decrease in bone mineralization. Nevertheless, bone fragility and fragility fracture in osteoporosis are influenced by multiple risk factors including genetic features, the level of weight-bearing physical activity, nutrition, smoking, body mass index (BMI), concurrent diseases, and medications.
3. Potential Clinical Application of FGF23 in Osteoporosis and CKD-MBD
Although current clinical studies are insufficient to support those findings and the hypothesis that FGF23 is independently associated with bone mineralization decline in the elderly and CKD-MBD, several clinical studies show that FGF23 has been a potential predictor for fragility fractures. High circulating FGF23 levels were discovered to be an independent risk factor for overall fragility fracture in elderly men [
29]. Furthermore, the three aforementioned studies discovered an independent association between elevated FGF23 and the incidence of fragility fractures in both moderate CKD and ESRD patients [
28,
34,
35]. Even though FGF23 levels had an independent negative relationship with BMD in postmenopausal women [
1,
4], it was not an effective discriminator between osteopenia/osteoporosis and normal bone mass [
4]. In addition, utilizing the FGF23 level for osteoporosis prediction in hemodialysis patients resulted in poor discrimination [
44]. Considering that various fragility fracture prediction models (e.g., the FRAX score, Q Fracture) are based on well-established clinical predictors (e.g., gender, BMI, CKD, alcohol, smoking, and corticosteroid use), there are currently no models that incorporate clinical predictors describing both systemic and local bone mineral homeostasis. The growing evidence indicates that FGF23 can represent the status of systemic bone mineral balance, renal function, and probably bone remodelling, thus the value-added of FGF23 on fragility fracture prediction awaits upcoming studies to explore its clinical potential to improve the prognostication of osteoporosis and CKD-MBD patients.
4. FGF23 Measurement in Routine Clinical Practices
Since none of the commercially available FGF23 assays have been validated for clinical use, FGF23 is not presently applicable for routine clinical practices. For the measurement of FGF23, four immunoassays are commercially available: Immutopics (1st and 2nd generation, San Clemente, CA, USA), Kainos (Tokyo, Japan), Millipore (Billerica, MA, USA), and DiaSorin (Saluggia, Italy). The majority of assays detect the intact 251 amino acid protein (iFGF23) by simultaneously recognizing epitopes on the N- and C-terminal domains located near the proteolytic cleavage site. Additionally, Immutopics provides an assay that quantifies both iFGF23 and the C-terminal fragment (cFGF23) using two antibodies against two C-terminal epitopes. iFGF23 is measured in picograms per milliliter (pg./mL), with a normal reference range of 11.7–48.6 pg./mL in a healthy individual, whereas cFGF23 is reported in relative units (RU) per milliliter, with a normal reference range of 21.6–91.0 RU/mL [
47]. Due to the possibility that iFGF23 may be degraded by protease enzyme or changed after venipuncture, two iFGF23 stability studies discovered decreasing FGF23 levels following an 8-h delay in centrifugation, but no evidence of deterioration after storing processed samples at −80 °C [
48]. Biological variability studies in healthy individuals revealed that iFGF23 levels have a diurnal variation that peaks in the early morning and gradually declines during the day [
47]. In comparison, the concentrations of cFGF23 could be slightly increased throughout the day [
49] and see no significant change after dietary or phosphate intake [
50]. Despite the stability and biological variability advantages of cFGF23, cFGF23 assays may be more applicable than iFGF23 assays, particularly for diagnostic and prognostic studies. In contrast, iFGF23 may outperform in representing the biological effects of FGF23 in etiognostic and therapeutic research because the c-terminal fragments might have counter-regulatory effects on the physiologically active FGF23 [
51]. The schematic summary of FGF23 production and its measurement are illustrated in
Figure 4.
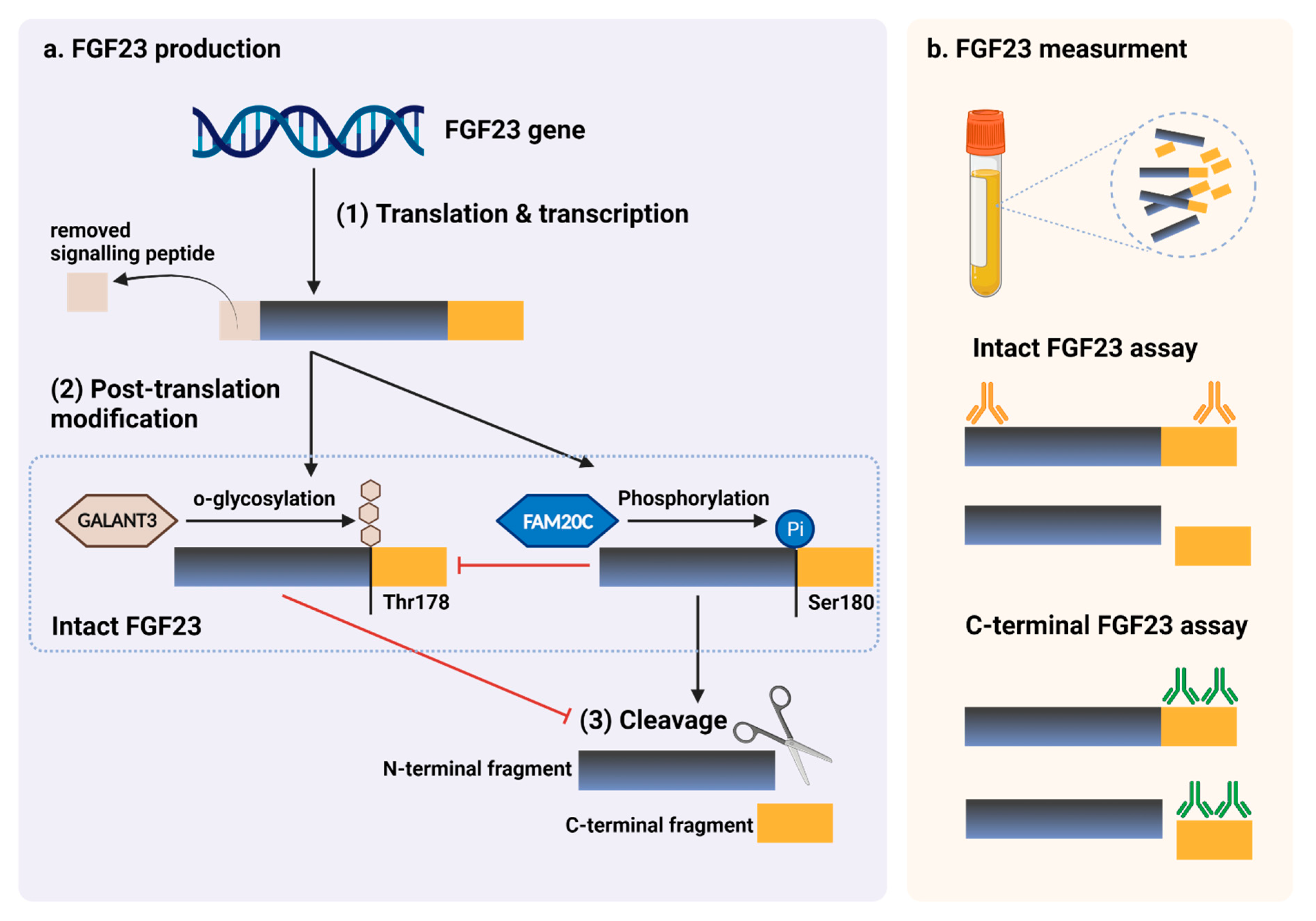 Figure 4.
Figure 4. FGF23 production and immunoassay measurements. (
a) After completed transcription and translation, FGF23 can be transferred to two post-translation modification pathways, including O-glycosylation with GALNT3 on Thr178, or phosphorylation by the extracellular serine/threonine protein kinase FAM20C at Ser180. O-glycosylation modification by GALANT3, stabilized form, can prevent intact FGF23 from cleavage. In contrast, phosphorylated FGF23 by FAM20C can be cleaved into N-terminal and C-terminal fragments within the osteocyte/osteoblast. These peptides, including full-length (intact) FGF23, N-terminal fragments, and C-terminal fragments, can be detected in the circulation. (
b) For C-terminal assays, detecting antibodies bind to C-terminus epitopes to detect both full-length FGF23 and its C-terminal fragments, whereas assays for intact FGF23 use antibodies to detect epitopes surrounding the FGF23 cleavage site for the detection of only full-length FGF23. This figure was generated with publication licensed by BioRender, Toronto, ON, Canada (Agreement number: DV237SONHF, 19 November 2021). Abbreviations: GALNT3, polypeptide N-acetyl galactosaminyltransferase 3; FAM20C, the extracellular protein kinase FAM20C; Ser, Serine; Thr, Threonine.
5. Conclusions
A pivotal role of FGF23 was found in local and systemic bone remodelling with supraphysiological levels causing abnormal bone formation, although any direct effect on osteoblasts remains unclear as well as a controversial links between FGF23 with osteoclastogenesis and bone resorption. Current evidence from clinical studies indicates that FGF23 could be a risk factor of bone fragility in CKD-MBD, but not a major contributor to age-related osteoporosis. An increased FGF23 level may represent an abnormal state of bone mineral homeostasis, but is not a direct indicator of decreased BMD. Since clinical studies, both in healthy elderly and in patients with impaired renal function, showed that elevated FGF23 levels were an independent risk factor of fragility fracture, a future predictive model for fragility fracture may incorporate FGF23 as a factor to represent bone mineral homeostasis status. FGF23 is putative factor in the fragility of CKD-MBD, less so in age-related bone loss; future elucidation of pathogenesis requires remodelling biomarkers, while gender differences need elucidation with respect to abnormal bone mineral homeostasis and reduced BMD as part of a future model of fragility risk factors.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23052500
 Figure 4. FGF23 production and immunoassay measurements. (a) After completed transcription and translation, FGF23 can be transferred to two post-translation modification pathways, including O-glycosylation with GALNT3 on Thr178, or phosphorylation by the extracellular serine/threonine protein kinase FAM20C at Ser180. O-glycosylation modification by GALANT3, stabilized form, can prevent intact FGF23 from cleavage. In contrast, phosphorylated FGF23 by FAM20C can be cleaved into N-terminal and C-terminal fragments within the osteocyte/osteoblast. These peptides, including full-length (intact) FGF23, N-terminal fragments, and C-terminal fragments, can be detected in the circulation. (b) For C-terminal assays, detecting antibodies bind to C-terminus epitopes to detect both full-length FGF23 and its C-terminal fragments, whereas assays for intact FGF23 use antibodies to detect epitopes surrounding the FGF23 cleavage site for the detection of only full-length FGF23. This figure was generated with publication licensed by BioRender, Toronto, ON, Canada (Agreement number: DV237SONHF, 19 November 2021). Abbreviations: GALNT3, polypeptide N-acetyl galactosaminyltransferase 3; FAM20C, the extracellular protein kinase FAM20C; Ser, Serine; Thr, Threonine.
Figure 4. FGF23 production and immunoassay measurements. (a) After completed transcription and translation, FGF23 can be transferred to two post-translation modification pathways, including O-glycosylation with GALNT3 on Thr178, or phosphorylation by the extracellular serine/threonine protein kinase FAM20C at Ser180. O-glycosylation modification by GALANT3, stabilized form, can prevent intact FGF23 from cleavage. In contrast, phosphorylated FGF23 by FAM20C can be cleaved into N-terminal and C-terminal fragments within the osteocyte/osteoblast. These peptides, including full-length (intact) FGF23, N-terminal fragments, and C-terminal fragments, can be detected in the circulation. (b) For C-terminal assays, detecting antibodies bind to C-terminus epitopes to detect both full-length FGF23 and its C-terminal fragments, whereas assays for intact FGF23 use antibodies to detect epitopes surrounding the FGF23 cleavage site for the detection of only full-length FGF23. This figure was generated with publication licensed by BioRender, Toronto, ON, Canada (Agreement number: DV237SONHF, 19 November 2021). Abbreviations: GALNT3, polypeptide N-acetyl galactosaminyltransferase 3; FAM20C, the extracellular protein kinase FAM20C; Ser, Serine; Thr, Threonine.