Spondias mangifera is a drupaceous fruit popular for its flavour and health advantages. There is little scientific knowledge about S. mangifera, despite its widespread usage in traditional medicine, in the North-Eastern region of India. Inhibiting the key carbohydrate hydrolysing enzymes is one of the strategies for managing diabetes. In the docking study, the main phytoconstituents of S. mangifera fruit, such as oleanolic acid, beta-sitosterol, and beta amyrin, show strong affinity for pancreatic α-amylase. These results imply that S. mangifera has α-amylase and α-glucosidase inhibitory properties and may be used as antidiabetic with antioxidant characteristics.
1. Introduction
Diabetes mellitus (DM) is a chronic endocrine system condition that results in hyperglycemia. It is characterized by long-term chronic hyperglycemia, which eventually leads to ultimate organ failure. DM has become a worldwide public health crisis affecting the lifestyles of the majority world population
[1]. DM is expected to impact 783 million people in the world by 2045, up from 537 million in 2021. It is one of the most severe health issues of the twenty-first century, according to the American Diabetes Association, and continues to be one of the fatal illnesses
[2][3]. The rising prevalence of diabetes is due to several circumstances, such as oxidative stress caused by the free radical generation that may cause β-cells in the pancreas to malfunction, decreased glucose tolerance, and insulin resistance. α-Amylase and α-glucosidase are two crucial key enzymes that break down carbohydrates and support the body to absorb them in the intestines
[4].
Antioxidants are a group of molecules that protect against ROS species in living things by competing with other molecules that are important for ROS oxidation
[5]. Antioxidants are preventive therapy that may help lessen the consequences linked with oxidative stress
[6]. Antioxidants are regarded useful therapeutic alternatives since oxidative stress involves a significant aspect in the development of diabetes mellitus. In addition, studies have shown that antioxidants and α-glucosidase inhibitors from natural sources, like fruits, may have anti-diabetic benefits, so these natural compounds have been getting a lot of attention from scientists around the world
[7]. Phytosterols are abundant in plants, vegetables, fruits, and nuts. β-sitosterol is the most common phytosterol, and several in-vitro and in-vivo investigations have shown that it has a variety of biological effects. The antioxidant function of β-sitosterol has been shown in several scientific investigations to work as a moderate radical scavenger chemically, as well as a membrane stabilizer physically
[8][9].
Spondias mangifera of the family Anacardiaceae is a glabrous tree. It grows to 10.5 m tall and has smooth ash-colored bark with a straight trunk and a distinct woody scent
[10]. The bark is used ethnopharmacologically as a tonic, a refrigerant, cure muscular and articular rheumatism, dysentery, and diarrhoea
[11]. Indian states, Punjab, West Bengal, Maharashtra, Assam, and Orissa, are the main places where this is cultivated as edible fruits
[12]. The leaves are aromatic, astringent, acidic, and are used to flavour food. While the juice is used to treat earaches
[13][14]. Sitosterol, stigmast-4-en-3-one, daucosterol, 24-methylene, lignoceric acid, and cycloartanone are found in the aerial sections of the plant. Other substances found in the fruits include β-amyrin and oleanolic acid
[15]. This plant’s ripe fruit juice is high in vitamins and can be employed as a nutraceutical agent
[16]. The root bark powder of the plant has been indicated for menstrual management, as well as anticancer, antihistamine, antipyretic, and antispasmodic properties
[17][18][19][20][21]. Additionally, the plant’s fruits and barks treat diabetes
[16]. In rats, methanolic extracts of stem heartwood had shown hepatoprotective action against carbon tetrachloride-induced liver toxicity
[22].
Phytomedicines derived from herbs and shrubs have been used in traditional healing procedures all across the globe since antiquity
[23]. Plant-based drugs or herbal compositions may play a key role in managing DM
[24]. Synthetic oral hypoglycemic medicines (Meglitinides, sulfonylureas, glitazones, gliptins, sulfonylureas, biguanides) and other currently available antidiabetic treatments have many side effects, such as sleepiness, anorexia, stomach pain, weight gain, etc.
[25]. Therefore, a comprehensive scientific examination of traditionally beneficial medicinal plants is required to investigate their antidiabetic efficacy using current experimental instruments and procedures. Computational molecular modelling has emerged as a significant sector in the natural product drug development process. In silico drug design tools enhance the discovery of novel medications from natural products. Computational modelling sheds light on the molecular recognition processes underlying the interaction between disease-related target macromolecules with naturally occurring drug-like substances
[26].
There is little scientific knowledge about S. mangifera, despite its widespread usage in traditional medicine, in the North-Eastern region of India. The antioxidant and anti-diabetic properties of different fractions of Spondias mangifera fruit extract from Indian geographical origin are examined. Furthermore, in silico using docking simulations experiments were conducted to confirm the in vitro enzymatic inhibitory capacity of phytocompounds, which was determined by the binding affinity and molecular couplings between phytochemicals and target enzymes. ADMET prediction studies were conducted to acquire a better knowledge of the association between activity and drug potency.
2. Anti-Diabetic Activity of Bioactive Compound Extracted from Spondias mangifera Fruit
S. mangifera fruits were extracted using a different polarity of solvents, including hexane, chloroform, and ethanol. The ABTS approach is quite practical and efficient for determining the scavenging activity of specified phytochemicals in plant extract fractions in a rapid, stable, and sensitive manner
[27]. Compared to other scavenging tests, the ABTS technique may be utilized in organic and inorganic solvent systems. This approach provides a more accurate assessment of lipophilic and hydrophilic free radical scavenging activity
[28]. The
S. mangifera fruit ethanolic fraction (EtOH-F) has superior inhibitory efficacy compared to the hexane (Hx-F) and chloroform fractions (Chl-F), suggesting that the antioxidants in the hexane and chloroform fractions have a low ability to scavenge free radicals.
During a free radical-initiated oxidative chain reaction, lipid peroxidation occurs when each successive lipid molecular is oxidized to the highest degree feasible. This chain reaction usually stops when the substrate runs out. Alternatively, joining two radicals to generate nonradical products or reacting with antioxidants offer easily donatable hydrogen for peroxyl radical abstraction. This test may be performed enzymatically (Fe/NADPH) or nonenzymatic (Fe/ascorbic acid). As researchers employed egg yolk like a substrate, it is possible to conclude that
S. mangifera can oxidize nonenzymatically. Researchers' investigation discovered that SMFEF exhibited superior inhibitory action compared to other tested fractions in the reducing power experiment. The color of the test solution varies from yellow to different colors of green and blue based on the extract’s reducing ability. Some previous researchers have reported a clear association between antioxidant activity and the reducing capacity of specific plant extracts
[29]. All extracts’ reducing power improves with increasing concentration, which is closely associated with their antioxidant activity. Therefore, these reducers must account for the antioxidant effects of extracts. Lipid peroxidation is a natural event that causes peroxidative loss at unsaturated lipids, resulting in lipid breakdown and membrane instability. Peroxidized lipids have been implicated in the pathophysiology of several illnesses and may act as a molecular basis of cell harm during pathological situations
[30]. As a sign of oxidative stress, lipid peroxidation is often assessed in terms of its catabolite malondialdehyde (MDA)
[31].
Diabetes is defined by abnormally high blood sugar levels, resulting in life-threatening consequences. It is characterized by elevated blood sugar levels, resulting in significant problems. Thus, the objective of diabetes treatment is to retain near-normal glycaemic control. Regrettably, there is no therapy or medication available in contemporary medicine that can effectively manage diabetes without causing adverse effects from insulin and oral hypoglycemic drugs. As a result, medicinal plants with antidiabetic effects may be helpful in the development of safer, more cost-effective antidiabetic medications. The anti-diabetic effect of
Spondias mangifera fruit extract fractions were investigated. α-Glucosidase is the enzyme responsible for the last step in the breakdown of carbs. It is found on the brush-border cell surface of the intestinal epithelium. α-Glucosidase inhibitors may slow the absorption of dietary carbs in the small intestine and decrease postprandial hyperglycemia, which might be a promising strategy for developing antidiabetic medicines. This is primarily utilized as a successful pharmaceutical treatment for hyperglycemia associated with type 2 diabetes in its early stages. A significant carbohydrate hydrolysis enzyme required for converting disaccharides and oligosaccharides to glucose is alpha (α)-glucosidase (GAA). This allows the small intestine to ingest the generated monosaccharides, resulting in an increase in blood glucose. GAA has been identified as the most important enzyme in the prevention and treatment of Type 2 diabetes. AGIs can help people with diabetes to maintain their blood glucose levels regular by slowing down the digestion of carbohydrates and reducing the absorption of monosaccharides. Consequently, screening AGIs is critical in preventing and treating type 2 diabetes (T2D). Currently, attempts are being made to screen AGIs derived from either organic substances or natural sources. As a result, screening for naturally occurring active chemicals with little adverse effects in natural goods as AGIs has gained increasing interest
[32].
Antioxidative systems in the human body, including enzymatic and non-enzymatic mechanisms, reduce the creation of reactive oxygen species, which are linked to a wide range of degenerative disorders, such as diabetes. When blood glucose levels rise, it binds to haemoglobin, causing reactive oxygen species to develop
[33].
S. mangifera fruit fractions strongly reduced haemoglobin glycosylation, as shown by increased haemoglobin concentrations that are shown by the formation of the glucose-haemoglobin complex, which increases free haemoglobin. The ethanolic fraction of
S. mangifera fruit (EtOH-F) inhibited glycosylation more effectively than the chloroform or hexane extracts. The dose-dependent percentage reduction of glycosylation was observed. Controlling the glucose concentration in the blood of a diabetic patient may help avoid the disease’s numerous consequences. The ability of mammalian species to maintain a stable plasma glucose content over an extended period of time under a range of dietary patterns is one of the most critical and finely controlled processes identified
[34].
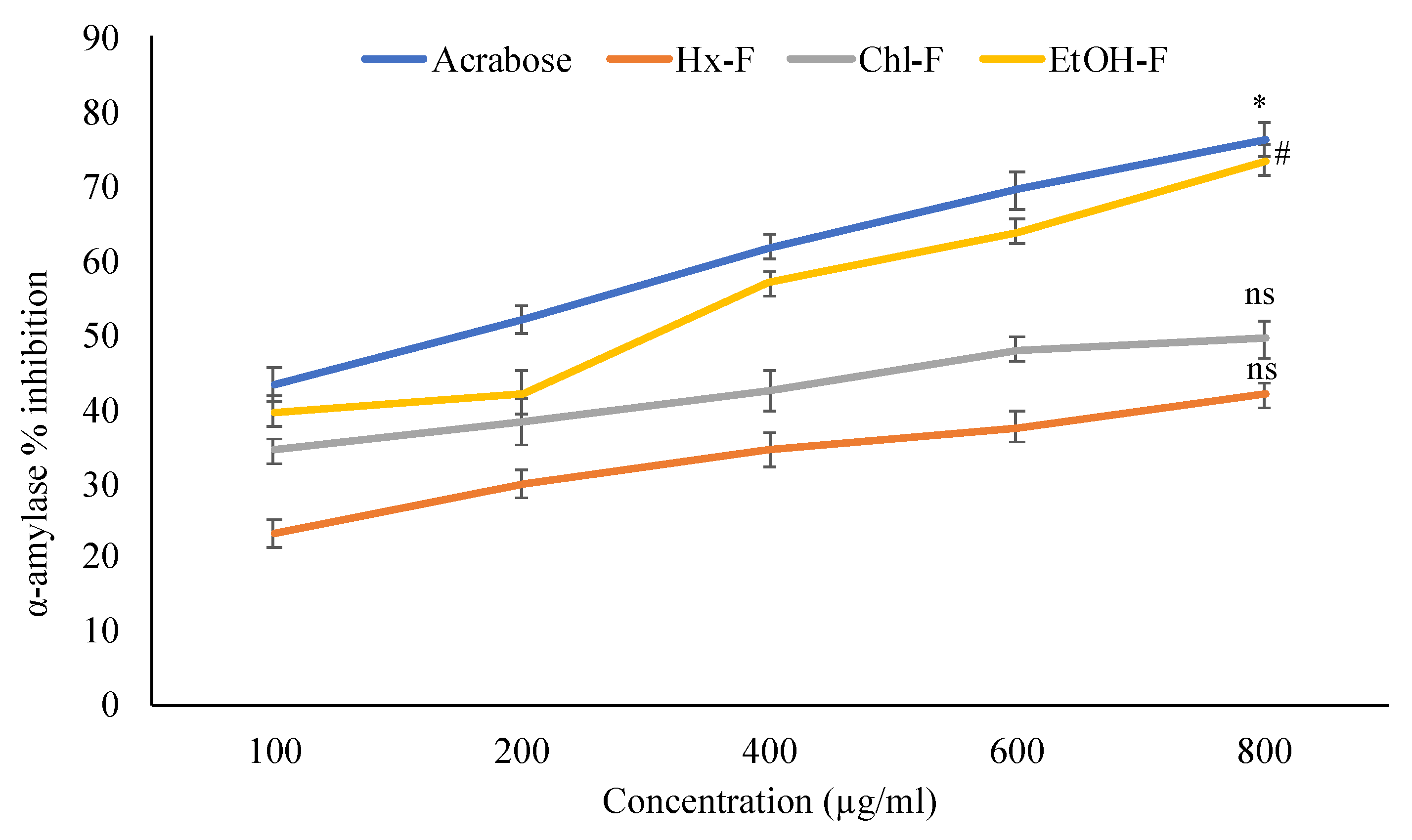
Alpha-amylase, one of the intestinal digestive enzymes, is essential for carbohydrate digestion.
S. mangifera has strong anti-diabetic action, as evidenced by in vitro α-amylase inhibitory tests (
Figure 1). A dose-dependent relationship was seen for the percentage inhibition at doses of 100, 200, 400, 600, and 800 g/mL of crude plant extracts. The % inhibition of ethanolic fraction at 800 µg/mL was 73.42 ± 2.01%, chloroform extract was 49.36 ± 2.39%, and hexane was substantially less at 41.83 ± 1.57%. The alpha-amylase hydrolyses large alpha-linked polysaccharides like starch and glycogen to produce maltose and glucose. Researchers' research discovered that compared to the standard acarbose 76.21 ± 2.18%, the ethanolic extract of
S. mangifera inhibited alpha-amylase activity significantly. Skeletal muscles consume glucose due to an increase of functional glucose transporter molecules in the cell membrane. In response to increased insulin production in the blood, leptocytes and myocytes control glucose transporting molecules, resulting in hypoglycaemia
[35].
Figure 1. Percentage inhibition of S. mangifera fruit extract fractions (SMFEF) on α-amylase (p < 0.05). Data are expressed as means ± SEM (n = 3), with a significance test for comparison with acarbose using ANOVA followed by Dunnet’s ‘t’ test. * p < 0.01, # p < 0.05 and ns p > 0.05 = non-significant.
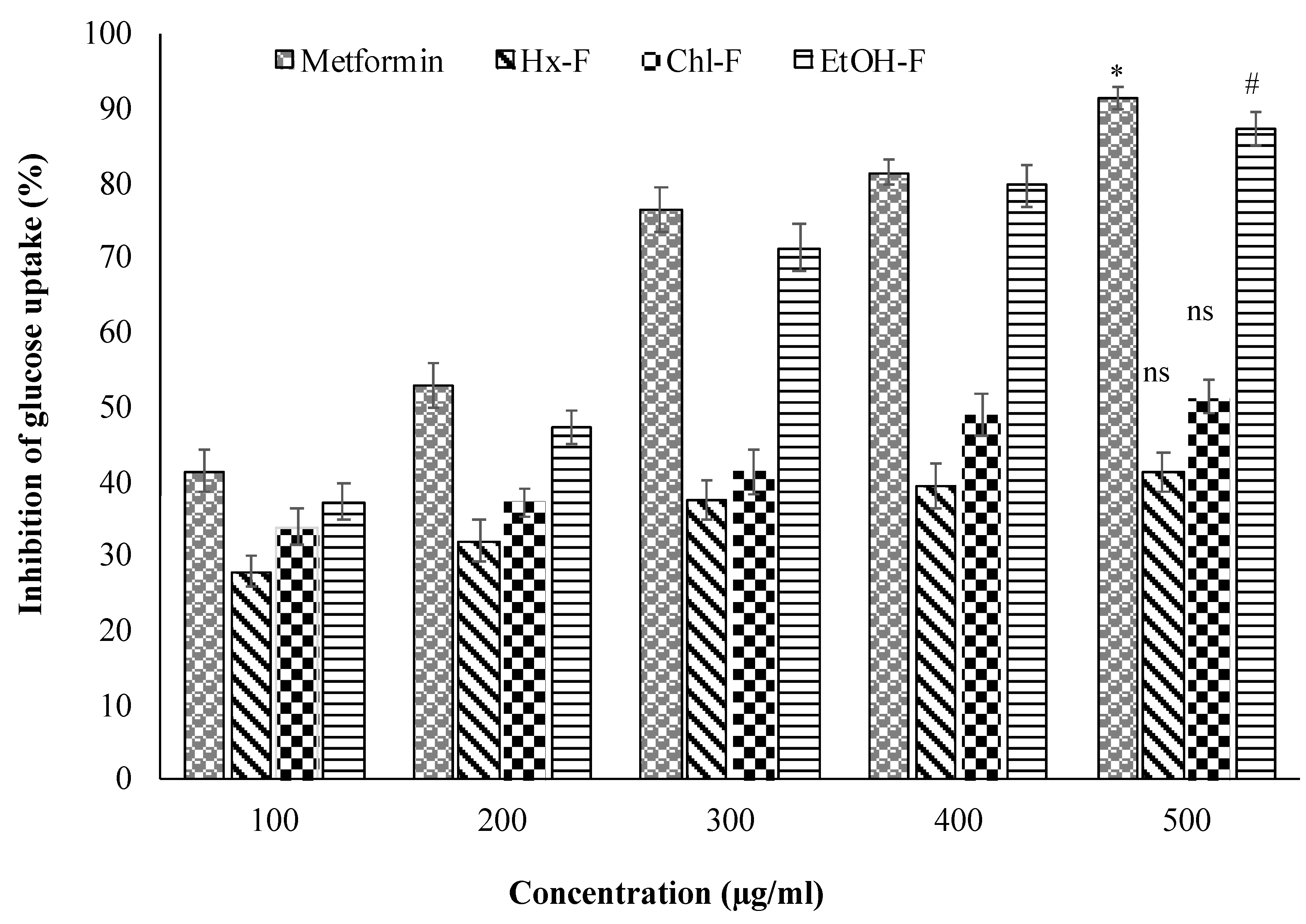
The current in vitro experiments demonstrated that the ethanolic extract had a favourable anti-diabetic effect. Figure 2 depicts the rate of glucose transfer through the cell membrane in the yeast cell system. Glucose transport in yeast (Saccharomyces cerevisiae) happens through facilitated diffusion. The glucose absorption of yeast cells was observed to increase dose-dependently upon treatment with these plant extracts. The S. mangifera fruit ethanolic fraction (EtOH-F) demonstrated considerably increased reactivity at all glucose concentrations, with the greatest increase seen at 500 g/mL glucose. Additionally, the results suggested that S. mangifera was highly effective in enhancing yeast cell glucose absorption when compared to the standard. Additionally, yeast cells may have a distinct glucose absorption mechanism than other eukaryotic or human body cells. Numerous factors, such as the glucose content within the cells or the subsequent glucose metabolism, might impact glucose absorption by yeast cells. For example, if the majority of the internal sugar is rapidly converted to other metabolites, the inner glucose level decreases, favouring glucose absorption into the cell. Similarly, glucose absorption by yeast cells in the presence of S. mangifera fruit extract fractions might result from enhanced diffusion and increased glucose metabolism.
Figure 2. Inhibition of glucose uptake by yeast cell of S. mangifera fruit fractions (SMFEF). Data are expressed as means ± SEM (n = 3), with a significance test for comparison with Metformin using ANOVA followed by Dunnet’s ‘t’ test. * p < 0.01, # p < 0.05 and ns p > 0.05 = non-significant.
Furthermore, when combined with α-amylase in their respective bound states in molecular docking studies, β-amyrin, β-sitosterol, oleanolic acid, and acarbose showed substantial similarity. Docking interaction of β-sitosterol compound (−5.68) showed the highest binding affinity. The dynamic behaviour and stability of β-sitosterol from MD simulation revealed the average RMSD values for acarbose-pancreatic alpha-amylase and β-sitosterol-pancreatic alpha-amylase complexes 0.17 (±0.03) nm and 0.18 (±0.02) nm, respectively. Thus, there was no observe any apparent differences between the ligand and reference RMSD indicating overall stability. The medication’s Drug Likeness is assessed by predicting the Absorption, Distribution, Metabolism, Excretion, and Toxicity (ADMET) features, which showed the pharmacokinetics of the selected compounds. The goal of computing ADMET profiles is to provide an early forecast of a compound’s in vivo performance so that its potential to become a drug may be assessed. According to Lipinski’s rule of five, it is governed by a complex balance of many molecular qualities and structural aspects, which impact the function of a component in a living organism. No more than one of the rules should be broken by a good drug contender
[36].
β-sitosterol is the most common phytosterol and has shown a variety of biological effects. The RP-HPLC analysis of EtOH-F of S. mangifera showed the percentage of β-sitosterol 1.21% ± 0.17% of total weight of extract (w/w). It will undoubtedly be fascinating to investigate the activities of natural extracts (including S. mangifera fruit extract fractions) that may aid in improved glucose absorption by muscle cells and adipose tissues. The extract efficiently binds glucose and transfers it through the cell membrane for additional metabolism. This research yields novel biological and chemical data for the S. mangifera, and it holds the promise of exciting discoveries and possibility of further research.
This entry is adapted from the peer-reviewed paper 10.3390/plants11040562