Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Polymer Science
|
Materials Science, Coatings & Films
Polyphenylsulfone (PPSU) membranes are of fundamental importance for many applications such as water treatment, gas separation, energy, electronics, and biomedicine, due to their low cost, controlled crystallinity, chemical, thermal, and mechanical stability. Numerous research studies have shown that modifying surface properties of PPSU membranes influences their stability and functionality. Therefore, the modification of the PPSU membrane surface is a pressing issue for both research and industrial communities.
- membranes
- modification methods
- polyphenylsulfone
1. Introduction
Polyphenylsulfone (PPSU) belongs to sulfone-family polymers that have been thoroughly studied for their potential applications in membrane science and technology. The state-of-the-art PPSU-based membranes show superior properties, including excellent thermal and mechanical stability, high chemical resistance, impact resistance, and hydrolytic stability [1,2,3,4,5,6,7,8]. This stability can be attributed to the difference in their backbone structure compared to other polymeric materials. However, the membrane morphological structure and properties are elaborated by the composition and operating conditions of a PPSU solution, including concentration, additives, solvent type, temperature, kinetic factors, the coagulation bath (phase inversion process), etc. [9,10,11,12,13,14,15,16]. Thermodynamics and kinetics play significant roles during membrane development [17,18,19]. Thermodynamics determines whether a PPSU polymer solution is stable or not. Kinetics plays a key role in the phase separation speed. Despite the aforementioned important properties of PPSU polymers, there has been a limited number of studies concerning the preparation of PPSU membranes [20,21,22,23]. However, these studies showed promising results in polymer applications. In particular, the membranes can be used for ultrafiltration, nanofiltration, and reverse and forward osmosis. At the same time, other polymer materials often prove more susceptible in terms of stability (chemical, thermal, and mechanical) and are often expensive [24,25,26,27,28,29,30].
One disadvantage of PPSU-based membranes is their hydrophobic nature, which leads to reduced surface energy. The latter causes poor antifouling ability by foulant pollutants in water. Two more disadvantages of the PPSU membrane are its low water permeability and high fouling ability. These two have limited its application in aqueous phase separation. A number of studies have concluded that membrane fouling is directly related to hydrophobicity and surface charge, as reviewed by several researchers, while the opposite has also been reported [31,32,33,34]. Membrane fouling is generally classified as organic fouling, inorganic fouling, or biofouling (nonpolar solutes, hydrophobic particles, microorganism, mineral scale). It can easily adhere to or accumulate on the membrane surface or plug membrane pores by hydrophobic interactions, hydrogen bonding, van der Waals attractions, and electrostatic interactions [14,35,36,37,38,39]. As a result, the membrane separation process becomes more complex, and its permeability and selectivity are reduced. The latter leads to an increase in operating costs, energy demand, and shorter membrane lifetimes.
Thus, the current trend is to improve PPSU membrane materials and structures and to get membranes with both good separation and antifouling performance. Controlling the membrane surface properties and structure has been a common goal for improving membrane separation performance (Figure 1). Achieving this goal is not an easy task. However, various types of inorganic and organic materials have been used to improve the characteristics of the PPSU membranes [28,40,41,42,43]. Several features, including the PPSU and additive concentrations, molecular weights, the miscibility characteristics, the compatibility with organic and inorganic materials properties, and the solvent type, can impact the performance of these additives.
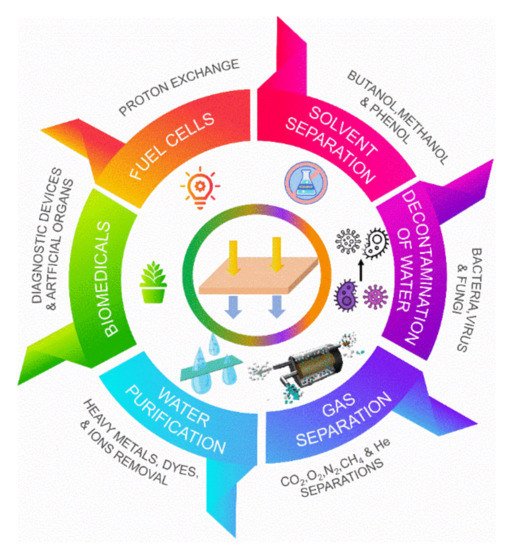
Figure 1. PPSU membranes significant environmental applications: water purification, gas separation, decontamination of water, solvent separation, fuel cell, and biomedical.
Multiple studies have reported the fabrication of PPSU-based membranes in different configurations, including flat sheet and hollow fibers [44,45,46,47,48]. However, to the best of our knowledge, there is no state-of-the-art report on PPSU-based membranes that summarizes their surface modifications and associated changes in performance. The current review serves to fill this research gap. More specifically, we bring together recent advancements in polymer and membrane development for the benefit of both academic and industrial researchers. We focus on various modification methods as well as performance evaluation. There are three main approaches for the modification of PPSU polymer or membranes with improved surface properties: (1) bulk modifications via sulfonation, amination, and chloromethylation; (2) blending with a synthetic polymer, inorganic nanomaterials, and biopolymer; and (3) surface modification via physical and chemical approaches (Figure 2).
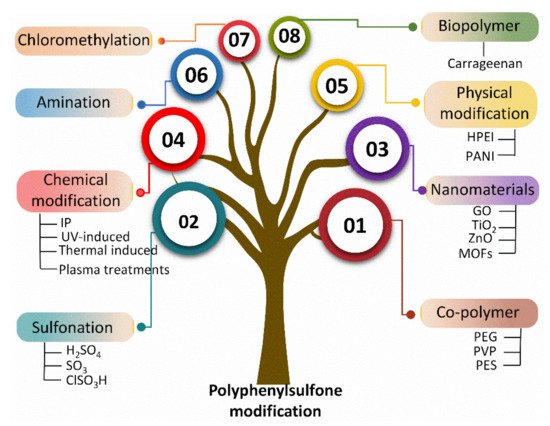
Figure 2. Modification techniques for PPSU utilizing various modifiers.
2. Polyphenylsulfone Characteristics
PPSU is the abbreviation for Polyphenylsulfone. Also known as PPSF, PPSU is a new member of the sulfone polymers family that has multiple attractive properties such as high-temperature performance, good chemical resistance (maintaining its original properties after being exposed to a harsh chemical environment pH at 1–13), outstanding toughness, corrosion resistance, chlorine tolerance, excellent colorability, and very good dimensional stability [49]. The polymer can be distributed in two different families depending on the level of the molecular organization of the constitutive chains at the microscopic level. Compared to other sulfone polymers, PPSU is an amorphous polymer. Therefore, it features very good creep resistance, isotropic thermal and mechanical properties, and transparency. PPSU consists of an aromatic unit (phenylene) chain with a sulfone group and a benzene ring, connected by an oxygen atom. Due to this conjugated structure, the rigidity of the material can be maintained, and it gives good liquidity [28,50]. Figure 3 shows the molecular structures of PPSU.
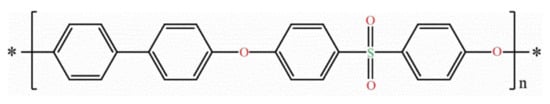
Figure 3. General structure of Polyphenylsulfone polymer.
The presence of the electronegative sulfone group results in sulfur being in its highest oxidation state. The latter brings excellent thermo-oxidative stability and easy functionalization. The surface of PPSU resin has a negative charge over a pH of 3 [30]. The existence of a biphenylene unit in PPSU resin significantly elevates the impact strength and reduces the notch sensitivity of the material. The latter results in strength at break (tensile) values greater than 75 MPa, a glass transition temperature of 288 °C, and a heat deflection temperature of 274 °C. Therefore, PPSU is expected to become the next widely used polymer in various applications, including membrane filtration, plumbing, food services, medical, aerospace, wire and cable, etc. On the other hand, the PPSU-based membranes that have been widely used in water applications have several drawbacks. The main drawback is related to its relatively hydrophobic nature. It reduces membranes’ permeability and makes them more susceptible to fouling during water treatment. Chemical cleaning is an essential step to sustain the membrane life. At the same time, high standards of water quality should also be met. Table 1 summarizes different methods that were used in the modification of PPSU membrane, modifier agents, and performance of modified membranes.
Table 1. Summary of frequently used methods for modification of PPSU membrane and performance.
| Description | Methods of Modification | Modifier Agents | Process of Membrane | Application | Performance | Ref. |
|---|---|---|---|---|---|---|
| Proton-conductive sPPSU membranes | Sulfonation | SO3 and (CH3)3 SiSO3Cl | Solvent evaporation | Electrochemical | (CH3)3SiClSO3 gave a homogeneous sPPSU with better control of the DS values as high as 1.0; asymmetric structure; high mechanical stability; proton conductivity about 55 mS/cm at 80 °C | [51] |
| Proton-conducting fuel cell sphPPSU membranes | Sulfophenylation | BuLi (metalating agent) and 2-sulfobenzoic acid cyclic anhydride | Vacuum dry | Fuel cells | sphPPSU showed DS values as 0.9; membranes have high thermal stability (300 and 350 °C); the proton conductivity about 60 mS/cm at 70 °C | [52] |
| PEI/PPSU sheet | Blending | PEI | Direct injection molding | Plasticization | PEI/PPSU blends are miscible; elasticity and yield stress changed linearly with PEI-rich blends composition | [53] |
| Proton exchange SPEEK/SiSPPSU membranes | Silylation and sulfonation; and blending | PhSiCl3 and H2SO4; SPEEK | Solvent evaporation | Fuel cells | SiSPPSU showed DS values as 2.0; exhibited high and stable conductivity values at 120 °C when dry (6.1 × 10−3 S/cm) and wet conditions (6.4 × 10−2 S/cm) | [54] |
| sPPSU-proton conducting membrane | Sulfonation | H2SO4 and ClSO3Si (CH3)3 | Sol-gel processes | Fuel cells | sPPSU reached the conductivity values as high as 1.1 × 10−2 S cm−1 at 130 °C | [55] |
| PPSU/PBNPI membrane | Blending | PBNPI | Solvent evaporation | Hydrogen separation | The gases H2, CO2 and CH4 permeability increased up to 50% | [56] |
| PPSU/PBNPI membrane | Blending; immersion method | PBNPI; p-xylylenediamine (crosslinking reagent) | Solvent evaporation | Gas permeation | O2 and N2 permeation rates of 23.2 and 22.42 | [57] |
| sPOSS/sPPSU composite proton exchange membranes | Blending | sPOSS | Dry | Fuel cells | sPOSS/sPPSU composites multilayered structure and reduce brittleness; conductivity 1 × 10−2 S cm−1 at 90 °C | [1] |
| Ionic exchange sPPSU/sPES membrane | Sulfonation; Blending | H2SO4; sPES | Solvent evaporation and dry | Fuel cells | The membrane surfaces show the smoother about 2 nm; stress–strain values 80 MPa and 7% | [5] |
| SPEEK/SiPPSU composite membranes | Silylation; Blending | SPEEK | Dry | Fuel cells | The presence of silicon enhances the temperature of loss of sulfonic acid groups; composites show superior behavior in terms of mechanical properties (higher elastic modulus and tensile strength) | [50] |
| PPSU/PEI membranes | Blending | PEI; PEG 200 | Wet phase inversion | Ultrafiltration | Asymmetric and spongelike structure; water contact angle decreases significantly upto 64° and EWC 59.37%; IEP shifted pH 8 and shown positive charge; flux 545.54 kg m−2 h−1; rejection 56% | [20] |
| sPPSU positively charged membrane | UV grafting | [2-(methacryloyloxy)ethyl]trimethyl ammonium chloride; diallyldimethylammonium chloride |
Nanofiltration; textile dyes | Spongelike morphology; MWCO 1627–1674 Da; PWP of 9–14 LMH bar−1; rejection of MgCl2 (95%) and Safranin O dye (99.9%) | [58] | |
| PPSU thin-film composite membrane | Oxygen plasma (pretreatment); surface modification | 2,5-bis(4-amino- 2-trifluoromethyl-phenoxy)benzenesulfonic acid; 4,4-bis(4-amino-2-trifluoromethyl-phenoxy)biphenyl-4,4-disulfonic acid | interfacial polymerization | Nanofiltration; dye removal | Water flux 63.9 and 71.3 L/m2 h; dye rejection 48–80% | [59] |
| sPPSU/sPES membranes | Sulfonation; Blending | H2SO4; sPES | Crosslinking; heat and dry |
Fuel cells | Maximum conductivity of 0.12 S/cm | [60] |
| sPPSU TFC membranes | Surface modification | MPD;TMC | Interfacial polymerization | Forward osmosis | Water flux up to 54 LMH with 8.8 gMH salt reverse flux under PRO mode | [61] |
| PPSU/PI solvent resistant membrane | Blending | PI | Phase inversion; solvent evaporation | Nanofiltration | Asymmetric structure with a dense skin layer; highest flux for alcohol and alkanes was achieved for a 50/50 wt.% blend; | [62] |
| PPSU/TiO2 nanocomposites membrane | Blending | TiO2 | Solvent evaporation | Biomedical | Nanocomposites shown active inhibition against E. coli and S. aureus bacteria with and without UV irradiation; the stiffness, strength, toughness, hardness and heat distortion temperature increases | [63] |
| Anion exchange PyPPSU membrane | Blending | 1-methyl-2-pyrrolidone | Solvent evaporation | Vanadium redox flow battery | Vanadium ions permeability (0.07 × 10−7–0.15 × 10−7 cm2 min−1); coulombic efficiency of 97.8% and energy efficiency of 80.2% | [64] |
| PPSU solvent resistant membrane | Blending | Cu-BTC | Phase inversion | Nanofiltration; methanol–dye separation | Improve tensile strength 29%; methanol flux 135 L m−2 h−1 | [65] |
| PPSU nanofibrous membrane | Blending | PEG 400 | Electrospinning | Wastewater treatments | Water contact angle 8.9°; porosity 72.4%; water flux 7920 L/m2h | [66] |
| PPSU membranes | Blending | sPPSU | Phase inversion | Ultrafiltration | Porosity 48%; MWCO 70 kDa; pure water flux 218 L m−2 h−1; FRR 79%; BSA rejection 85% | [49] |
| sPPSU/PIM-1 membrane | Blending | sDCDPS; PIM-1 | Slower solvent evaporation | Gas Separation | The tensile strength up to 72 MPa and extension at break 3.5%; the gas separation performance above the Robeson upper bounds for O2/N2, CO2/N2, CO2/CH4 | [67] |
| PPSU/FAC composite membrane | Blending | FAC | Phase inversion | Phenol filtration | Fragmented surface and spongy porous linkages; contact angle 43.8°; porosity 30%; pure water flux 26 Lm−2 h−1, phenol rejection 96.4% | [68] |
| MgO/sPPSU/PPSU membranes | Blending | MgO; sPPSU | Phase inversion | Ultrafiltration; Oil separation | Porosity 65% and MWCO 70 kDa; contact angle 48°; FRR 85% and HA rejection 63% and castor oil rejection 99% | [69] |
| PPSU/Cu-BTC solvent resistant nanofiltration | Blending | Cu-BTC | Phase inversion | Nanofiltration; dye and methanol separation | Contact angle 61°, and porosity 62%; Flux 19 L/m2 h and rejection of methanol 93% | [70] |
| sPPSU proton exchange membrane | Sulfonation; Blending | H2SO4 | Solvent evaporation | Fuel cells | Conductivity of 0.1 S/cm and power density of 471 mW/cm2 at 80 °C | [71] |
| PPSU membrane | Blending | PVP; PEG; Tween 80 | Phase inversion | Ultrafiltration | Water flux 148 L/m2 h; BSA rejection increased from 53.2% to 81.5% | [30] |
| sPPSU asymmetric membranes | Sulfonation; Blending | TMSClS | Phase inversion | Ultrafiltration | Decomposition temperature at 510 °C; contact angle 33°, and porosity 51%; FRR 70% | [72] |
| sPPSU/f-SWCNTs mixed-matrix membranes | Sulfonation; Blending | 3,3′-disulfonated 4,4′-dichlorodiphenyl sulfone; f-SWCNTs | Phase inversion | Gas separation | Enhanced the permeability for N2, O2, He, and CO2 and the selectivity for O2/N2 and O2/CO2 | [73] |
| Porous PPSU membrane | Blending | Carrageenan | Phase inversion | Ultrafiltration | Contact angle 43° and porosity 78%; zeta potential −24 mV at pH 7; permeability increased up to 29 Lm−2 h−1 bar−1 | [74] |
| PPSU/GO mixed matrix membrane | Blending | GO; PEG1000 | Phase inversion | Ultrafiltration | Hydrophilicity and the thermal stability improved; pure water flux 132 L·m−2·h−1 and the rejection 96.8% | [28] |
| PPSU/Zeolite mixed matrix membrane | Blending | Fe-ZSM-5; Cu-ZSM-5 | Phase inversion | Organic compounds removal | Surface roughness increased (Ra- 18.52 nm); zeta potential about −57.2 mV at pH 7; water flux of 62 L·m−2·h−1, lignin rejection up to 88.5% | [31] |
| PPSU/BiOCl-AC membrane | Blending | BiOCl-AC; PVP | Phase inversion | Ultrafiltration; oil separation | Asymmetric structures with thick top layer; contact angle 67°; pure water flux 465 L·m−2·h−1; rejection diesel fuel 80% and 90% of crude oil | [42] |
| Alkali resisting PPSU membrane | Blending | PVP- 10, 55, 360, and 1300 kDa | Phase inversion | Ultrafiltration | Asymmetric and fingerlike structure; Tensile strength upto 2.53 MPa for 10 kDa; MWCO ranged from 2 kDa to 175 kDa; pure water flux 69 L·m−2·h−1; better anti-alkali property in NaOH solution (pH = 13) | [13] |
| HBE–MMT/PPSU nanocomposite membrane | Blending | Functionalized montmorillonite | Phase inversion | Water treatment | Contact angle 53.6°; pure water flux about 380 L·m−2·h−1 at 5 bar; rejection of salt 40–50% | [75] |
| Polyamide TFN PPSU membrane | Blending; Surface modification |
GO (support layer); PIP and TMC | Interfacial polymerization | Nanofiltration; l kinetic hydrate inhibitor (KHI) removal | KHI rejection of 99% and permeation flux of 32.7 L/m2 h (at 9 bar and feed concentration of 0.5 wt.% KHI) | [76] |
| sPPSU/TiO2 mixed matrix hollow fiber membranes | Blending | TiO2 | Phase inversion | Ultrafiltration | Pure water flux 60 L·m−2·h−1; contact angle 67°; rejection of BSA 91% | [77] |
| PPSU membrane | Blending | PEG 400; PEG 20000 | Phase inversion | Filtration of aqueous media | Porosity 72%; tensile Strength at Break 7.75 MPa and elongation at Break 50.14%; Pure water flux 19 L·m−2·h−1 (PEG400) and 183 L·m−2·h−1 (PEG20000); 100% turbidity rejection | [10] |
| PPSU membrane | Blending | PEG 400; PEG 2000; PEG 6000; PEG 20000; PEG 35000; PEG 40000 | Phase inversion | Ultrafiltration | Contact angle 50° to 90°; pure water flux of 486 Lm−2 h−1; human serum albumin rejection 90% | [78] |
| Ionic crosslinked sPPSU membrane | Surface modification | HPEI | Coating | Nanofiltration; organic solvent filtration | Ethanol permeability 1.47 L m−2 h−1 bar−1; rejection of 99.9% to Rose Bengal dye | [79] |
| High-Flux PPSU membranes | Blending | PEG 6000–40000 | Phase inversion | Ultrafiltration | Pure water flux 500–1000 L m–2 h–1 at 0.1 MPa; 90% rejection of human serum albumin (PEG20000) | [80] |
| PA-MOF/PPSU-GO TFN membrane | Blending; Surface modification | GO (support layer); MOF; PIP and TMC | Interfacial polymerization | Nanofiltration | Permeate flux 59.9 L/m2·h; KHI rejection 96%; FRR 97.8% and an excellent long-term stability | [81] |
| sPPSU/PBI membrane | Blending; crosslinking | PBI; DBX (crosslinker) | Heat and solvent evaporation | Nanofiltration; organic solvent removal | Permeability 11.8 Lm−2 h−1 bar−1; rejection of tetracycline 97%. | [82] |
| Double crosslinked sPPSU/PBI membrane | Blending; crosslinking | PBI; DBX (crosslinker) | Heat and solvent evaporation | Nanofiltration; hydrogen purification | H2 permeability of 46.2 Barrer and a high H2/CO2 selectivity of 9.9 at 150 °C | [83] |
| Amine functionalized PPSU membrane | Amination; Blending | SnCl2; HNO3 | Phase inversion | Nanofiltration; dye removal | Pore size of 0.72 nm; positively charged active layers; contact angles 31°; pure water flux ∼54 Lm−2 h−1; CaCl2 and AlCl3 multivalent salts rejection 89% and 93.5%; crystal violet dye rejection > 99% | [84] |
| High-performance PPSU/sPANI membrane | Blending | sPANI | Nonsolvent induced phase separation | Ultrafiltration | Contact angle was 57°; porosity 81%; BSA adsorption value of 3.6 μg/cm2; water flux of 260 L/m2 h; BSA rejection 95% | [40] |
| PPSU/carboxylated GO nanocomposite membrane | Blending | Carboxylated GO | Phase inversion | Nanofiltration; heavy metal removal | Surface charge of −70 mV; flux of 27 L m−2 h−1; rejection of As(V) 96%, Cr(VI) 93%, Zn2+(81%), Cd2+ (74%), Pb2+ (73%) | [85] |
| sPPSU membrane | Sulfonation | H2SO4 | Phase inversion | Ultrafiltration; heavy metal and protein separation | Water flux of 190.33 Lm−2 h−1 and FRR of 86.56%; protein rejection of 66.3%, 74.0% and 91.2% for trypsin, pepsin, and BSA; Cd2+ and Pb2+ ions rejection of 75.2% and 87.6%; |
[86] |
| PPSU/carboxylated GO nanocomposite membrane | Blending | Carboxylated GO | Phase inversion | Ultrafiltration; Antimicrobial and antifouling | Bacteriostasis rates of 74.2%,81.1% and 41.9% against E. coli, P. aeruginosa and S. aureus; FRR 95.3% | [87] |
| Porous PPSU/sPEEK membrane | Blending | sPEEK | Solvent evaporation | Vanadium flow batteries | Contact angle 47°; tensile strength 2.78 MPa; proton conductivity of 14.3 mS cm−1 at 15 °C |
[88] |
| PPSU/SnO2 mixed matrix hollow fiber membrane | Blending | SnO2 | Vacuum evaporation | Ultrafiltration; dyes removal | Contact angle 63°; porosity 84%; pure water flux 362.9 L/m2 h; dyes rejection about >94% for RB-5, and >73% for RO-16 | [89] |
| PPSU/CuO/g-C3N4 membrane | Blending | CuO/g-C3N4 | Nonsolvent induced phase inversion | Ultrafiltration; antifouling and protein separation | Smooth surfaces Ra-9.8 nm; increase pores on the top layer as well as in the sublayer; contact angle 48°; water flux 202 L/m2h; BSA protein rejection 96%; FRR 79% | [90] |
| Super-hydrophilic PPSU TFC membrane | Surface modification | MPD and TMC | Electrospun; plasma treatments; interfacial polymerization | Forward osmosis | Contact angle 0°; Osmotic water flux 14 L/m2h | [91] |
| PPSU hollow fiber membranes | Blending | CA; CAP | Dry-wet spinning | Ultrafiltration; arsenic removal | Contact angle 60° and 43°; arsenic removal 34% and 41%; pure water permeability 61.47 L/m2h bar and 69.60 L/m2 h bar; FRR 88.67% | [92] |
| PPSU/silver-hydroxyapatite nanocomposite membrane | Blending | silver-hydroxyapatite | Phase inversion | Ultrafiltration; organic matter removal | Porous and honeycomblike structure; contact angle 60°; rejection 89% | [93] |
| Proton exchange sulfonated PPSU/PSU membrane | Sulfonation | Trimethylsilyl chlorosulfonate; | Vacuum dry | Fuel cells | Proton conductivity 34.1 mS cm−1 at 70 °C; power density of 400 mW cm−2; current density of 1100 mA cm−2 | [35] |
| PPSU/Ag-MWCNTs nanocomposite membrane | Blending | Ag-MWCNTs | Phase inversion | Nanofiltration; ion removal and antibacterial activity | Zeta potential −78 mV; contact angle 49°; porosity 73%; rejection of Na2HAsO4 99.5% and Na2Cr2O7 100% | [87] |
| PPSU/MWCNTs membrane | Blending | MWCNTs | Phase inversion | Ultrafiltration; heavy metals removal | Dense skin layer on top and a porous supportive sub-layer; surface roughness Ra 21 nm; contact angle 61°; porosity 50%; flux 186 L/m2 h rejection of Pb2+ (>98%), Hg2+ (>76%) and Cd2+ (>72%) | [94] |
| PPSU/ZnO nanocomposite membrane | Blending | ZnO | Phase inversion | Nanostructured- hybrid membranes; anionic dye; antimicrobial; wastewater treatment | Pore size 0.75 nm; zeta potential –65.7 mV at pH 7; methyl orange dye rejection 98% with a water flux 19 L/m2h; antibacterial activity of E. coli (6.2) and S. aureus (6.8) | [95] |
| Hydrophilic PPSU membranes | Blending | 1,2-propandiol; PVP | Nonsolvent induced phase separation | Ultrafiltration | Contact angles of 46.4°;Water flux 674 kg m−2 bar−1h−1 | [96] |
| PPSU/PES/SiO2 nanocomposite membrane | Blending | PES; SiO2 | Vapor induced phase separation; nonsolvent induced phase separation | Ultrafiltration | Water flux 76.65 L/m2·h; BSA retention of 82.01%; | [97] |
| Silica filled PPSU/PDMS Composite Membranes | Surface modification | PDMS; Silica | Coating | Biobutanol Separation | Weight loss starts from 400 °C; contact angle ∼130°; flux 536 g. m−2 h−1; butanol separation factor 30.6 | [36] |
| PPSU/PANI hollow fiber membrane | Blending | PANI | Dry-jet wet spinning | Humic acid removal | Zeta potential −16 mV at pH 9; Water flux 127 L/m2h; Humic acid rejection 98%; | [98] |
| Proton exchange sPPSU membrane | Sulfonation | H2SO4; CNDs (crosslinker) | Vacuum dry | Fuel cells | Proton conductivity 10−2 S/cm at 120 °C. | [99] |
| PPSU/Al-MOF mixed matrix membrane | Blending | Al-MOF | Phase inversion | Ultrafiltration,; dye separation; antifouling | Contact angle 63°; surface roughness Ra 21.9 nm; pure water flux 47 L·m−2·h−1; FRR 93%; rejection of organic dye methyl violet 93.8% | [100] |
| PPSU/CA/ZrO2 hollow fiber membranes | Blending | CA; ZrO2 | Dry-wet spinning | Arsenic Removal | Surface roughness Ra 43 nm; contact angle 48°; permeability of 89.94 L/m2h bar; removal of arsenic 87% | [45] |
| PPSU/CA hollow fiber membrane | Blending | CA | Dry–wet spinning | Removal of dyes | Permeability 64.47 L/m2 h bar; removal of Reactive black 5 dye 95% | [101] |
| PPSU/Zn-MOF composite membrane | Blending | Zn-MOF | Phase inversion | Ultrafiltration; antifouling | Asymmetric structure and dense microporous active skin layer; surface roughness Ra 13.88 nm; porosity 72%; tensile strength 7.9 MPa; permeability 33 L m−2 h−1 bar−1; FRR 98% | [102] |
| PPSU/CA/ZnO-MgO hollow fiber membrane | Blending | CA; ZnO-MgO | Dry–wet phase inversion | Arsenic removal | contact angle 60°; permeability 69.58 L/m2h bar; arsenic rejection 81.31%; FRR 91% | [103] |
| PANI coated PPSU Membranes | Surface modification | PANI | Coating | Dye separation; antibacterial activities | Surface roughness Ra-3.15 nm; contact angle 55°; zeta potential −1.7 mV at pH 6; permeability 53 L·m−2·h−1·bar−1; rejection of methylene blue dye 96%; bacteriostasis of E. coli 95% and S. aureus 88% | [104] |
3. Bulk Modification
3.1. Polyphenylsulfone Sulfonation
Sulfonation is defined as an aromatic electrophilic substitution reaction used to attach the sulfonic acid group to the molecule of an organic compound via a chemical bond, wherein an ortho positions the aromatic ring in place of the hydrogen atom. It is attributed to the fact that this electron-donating oxygen atom activates the ortho position [35,40,44,105,106,107,108]. Sulfonation is the accumulation of sulfonic groups at the aromatic backbone (including a phenyl and a sulfone unit as part of the backbone) of PPSU due to electron-donating substituents enhancing sulfonation. However, electron repulsing substituents have the opposite effect. PPSU is difficult to sulfonate because of the electron-withdrawing effect of the sulfone linkages that deactivate the adjacent aromatic rings for electrophilic substitution [9,51,72,109,110,111]. Moreover, its sulfonation requires stronger reagents and/or longer times. However, sulfonated PPSU features increased hydrophilicity and proton conductivity in the presence of sulfonate groups in the polymer chain. The latter, however, can introduce negative charges.
The sulfonation of the aromatic backbone of the polymer is carried out before membrane fabrication using sulfonating agents. There are three groups of sulfonating agents that do not cause polymer chain degradation. Sulfuric acid (H2SO4), sulfur trioxide (SO3), chlorosulfonic acid (ClSO3H), fluorosulfonic acid (FHO3S), amidosulfonic acid (H3NO3S) and its complexes, halogen derivatives of sulfuric acid, etc., form the first group and are derived from sulfur trioxide. They are referred to as electrophilic reacting agents and are most frequently used to sulfonate aromatic compounds. The second group includes nucleophilic agents such as sulfites (SO32−), hydrogen sulfites (HO3S−), and sulfur dioxide (SO2), which react with halogen derivatives and unsaturated compounds containing multiple bonds. The third group contains radically reacting agents. In particular, it includes sulfuryl chloride (SO2Cl2) and blends of gases (sulfur dioxide and chlorine (SO2 + Cl2), sulfur dioxide, and oxygen (SO2 + O2)). Sulfonation of polymer can be completed via either a heterogeneous reaction or a homogeneous reaction in hydrocarbons or chlorinated solvents.
The polymer sulfonation method works for most reagents. In the sulfonation protocol, the dried polymer is dissolved in a sulfonating agent and stirred at approximately 50 °C to produce a homogeneous solution in a nitrogen atmosphere. After the reaction, the solution is poured into a large volume of ice-cold deionized water under continuous stirring. As a result, a white precipitate is obtained. After standing overnight, the white precipitate is filtered and washed several times with cold deionized water to attain neutral pH level. The sulfonated polymer is then dried in a vacuum at room temperature [5,55,86,107].
A typical procedure is described by Hartmann–Thompson et al. [112] PPSU was added to dichloromethane (CH2Cl2), mixed, and placed in an ice bath on a stirring plate. Next, the solution was cooled to 10 °C under agitation. ClSO3H was added by drops over a one-hour period while stirring continuously. In the next step, acetic anhydride was added to the mixture by drops. The reaction was then allowed to continue for a period of time while stirring and maintaining the temperature. The reaction was stopped by gradually pouring the reacted solution into an ice-deionized water mixture. The resultant precipitate was recovered by pouring and washed repeatedly with deionized water until the wash water had a neutral pH. The PPSU was subsequently dried in an oven. Minor variations have been reported by other researchers, and a generalized overview of the sulfonation reaction scheme is shown in Figure 4a [107,113].
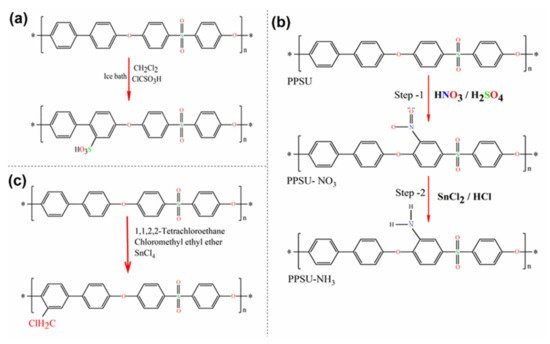
Figure 4. Synthesis of polyphenylsulfone. Reaction pathways: (a) sulfonation; (b) amination; (c) chloromethylation.
Liu et al. [9] demonstrated the sulfonation of PPSU random copolymers with various disulfonation levels. These were prepared via the direct polymerization method by aromatic nucleophilic substitution copolymerization. The reaction mechanism for this direct sulfonation has also been outlined. Karlsson et al. [52] prepared SPPSU by an anionic modification using n-butyllithium (BuLi) as a metalating agent. The preparation was performed via a one-pot synthesis in a reactor equipped with a gas inlet/outlet. In this method, the PPSU was dissolved in anhydrous tetrahydrofuran (THF) and cooled. The polymer solution was carefully titrated with a solution of BuLi until a faint reddish color was achieved. Subsequently, an amount of 2-sulfobenzoic acid cyclic anhydride corresponding to a twofold excess in relation to the lithiated sites of the polymer was quickly added in the form of a fine degassed powder and immediately dissolved and quenched the lithiated sites. Next, SPPSU was precipitated to remove the reactant residues via isopropanol. The precipitate was then filtered and dried in a vacuum and characterized by combining FTIR, 1H NMR, and 13C NMR spectroscopy. Licoccia and coworkers [55] followed the same methodology for sulfonated PPSU with H2SO4 and ClSO3Si(CH3)3.
H2SO4 is a low-cost sulfonating agent. However, it causes degradation of the main polymer chain when the reaction temperature is too high, or the reaction time is too long. This degradation may change the mechanical resistance of the membrane, therefore compromising its use in industrial applications. Among other reagents, the sulfonated PPSU with ClSO3Si(CH3)3 showed better sulfonation control when compared to the one sulfonated with SO3 [51,72]. However, SO3 has a drawback in that side reactions may occur. Moreover, the reaction is heterogeneous because when a part of the polymer reacts with SO3, it becomes insoluble in an apolar solvent. The rest of the reaction must be carried out in a dispersed system and not in a homogeneous solution. To solve the heterogeneity problem, ClSO3Si(CH3)3 can be used.
3.2. Polyphenylsulfone Amination
In the core of sulfonic groups, the existence of an amine group can enhance the physicochemical properties of the membrane compared to the others formed by segments. This process involves a similar substitution reaction but with amine groups as substituents. PPSU can be simply nitrated to almost one nitrogen atom per reproductive unit via reactions with strong bases. The latter is due to the sulfonic group having a strong activation effect on the nitration process. In this nitration reaction, the acidic ortho-to-sulfone hydrogens are replaced with nitrogen atoms that result in a positive charge of the carbon atom in the phenylene unit. Arumugham et al. [84] used a two-step method for the amination of PPSU. The reaction scheme for amine-functionalized PPSU is shown in Figure 4b. In the first step, nitration of PPSU was performed with nitric and sulfuric acid. It resulted in nitrogen atoms being placed in the ortho position. In the second step, the intermediate was aminated using tin chloride with hydrochloric acid. A similar polymer amination procedure has also been applied by other researchers [114,115].
Aminated polymer synthesis was followed by the reaction in a nitrogen atmosphere. PPSU (10 g) was added to a mixture of sulfuric acid and nitric acid (1/4 mixing ratio, 200 mL) and then stirred for 2 h at 25 °C to produce nitrated PPSU (PPSU-NO2). The resulting product was washed with five times the deionized water (300 mL) and dried in a vacuum oven at 30 °C for 24 h. To synthesize aminated PPSU, the tin chloride (20 g, 0.105 mol) in hydrochloric acid solution (20 g, 37%) was added in 60 mL of ethanol in a circular bottom flask. The flask was kept at 70 °C and then allowed to stir for 10 min. Subsequently, the synthesized PPSU-NO2 (5 g) powder was added slowly to the flask. The color of the solution transformed from yellow to dark brown, indicating the progress of the reaction. The reaction was further carried out for 4 h with stirring at 70 °C. Afterward, the reaction mixture was poured into 400 mL of deionized water for precipitation. Finally, PPSU-NH2 was separated, washed with deionized water, and dried in a vacuum oven at 80 °C for 12 h [84]. Considered amino groups have a significant effect on the surface charge and hydrophilicity of the PPSU polymer, as was shown in ion exchange capacity and water absorption measurements.
3.3. Polyphenylsulfone Chloromethylation
PPSU is a polymer that has no functional groups for further chemical modifications. However, the chloromethylation reaction of aromatic polymers is of particular interest to researchers and includes attaching functional groups onto aromatic ring-like chloromethyl. Currently, chloromethylation is actively investigated, both theoretically and experimentally, in the context of the procurement of precursors for functional membranes. Chloromethyl generally provides higher flexibility with time since it can easily interact with any kind of amines. Once a functional group is attached to the aromatic ring, further reactions can occur, including immobilization of compounds for enhanced hemocompatibility resulting in antifouling capability [116]. Zhang et al. [64] carried out the chloromethylation of PPSU and cast anion exchange membranes from the resultant chloromethylated PPSU. Chloromethylation of PPSU was performed following the one-step procedure. In a typical reaction shown in Figure 4c, the PPSU was dissolved in tetrachloroethane, and then tin tetrachloride and chloromethyl ethyl ether were added to the solution. The reaction mixture was heated, and the temperature was maintained.
After the desired reaction time elapsed, the reaction mixture was precipitated in excess ethanol, and the chloromethylated PPSU polymers were isolated by filtration. The polymer was purified by dissolution in chloroform and precipitation with ethanol and then dried in a vacuum oven. Chloromethylation of the polymer was not easy to control, and the number of chloromethyl groups attached to the polymer could be very small. The latter affected the properties of the membrane. The reaction mixture often produced a gel if it was not properly controlled by adjusting the temperature and the reaction time. Most of the studies considered chloromethylation of the polymers with the system made from trioxane and chlorotrimethylsilane as an agent of chloromethylation in the presence of tin tetrachloride [117,118]. The chloromethylation of polymers was dependent primarily on the kind of chloromethylating agent, the polymer structure, the type and amount of solvent, the catalyst, and other parameters of the reaction.
This entry is adapted from the peer-reviewed paper 10.3390/membranes12020247
This entry is offline, you can click here to edit this entry!
