If a bacterium has motility, it will use the ability to survive and thrive. For many pathogenic species, their motilities are a crucial virulence factor. The form of motility varies among the species. Some use flagella for swimming in liquid, and others use the cell-surface machinery to move over solid surfaces. Spirochetes are distinguished from other bacterial species by their helical or flat wave morphology and periplasmic flagella (PFs). It is believed that the rotation of PFs beneath the outer membrane causes transformation or rolling of the cell body, propelling the spirochetes. Interestingly, some spirochetal species exhibit motility both in liquid and over surfaces, but it is not fully unveiled how the spirochete pathogenicity involves such amphibious motility.
- bacteria
- motility
- spirochete
- Leptospira
- leptospirosis
- virulence factor
- crawling
- flagella
1. Introduction
2. Leptospirosis
3. Morphology and Motility of Leptospira
3.1. Cell Morphology
Leptospira spp. have two PFs (1 PF/cell end) within a thin (~150 nm in diameter), long (~20 μm in length), and short-pitch helical cell body (~700 nm in wavelength). The extracted PFs from the cell body exhibit a coiled shape, thus giving the cell ends curvature. The cell-end morphology depends on the gyration direction of the cell ends: Gyrating counterclockwise (CCW, viewing the cellular tip as indicated by thick black arrows in the cartoon of Figure 1) and clockwise (CW), the cell end form a “hook” shape and “spiral” shape, respectively (Figure 1) [15].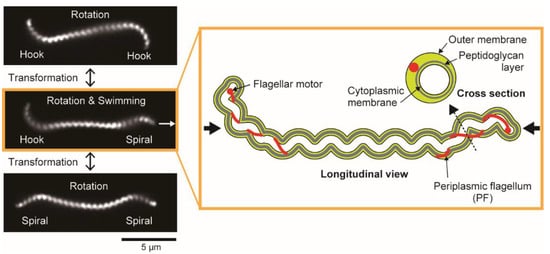
3.2. Swimming
3.3. Crawling
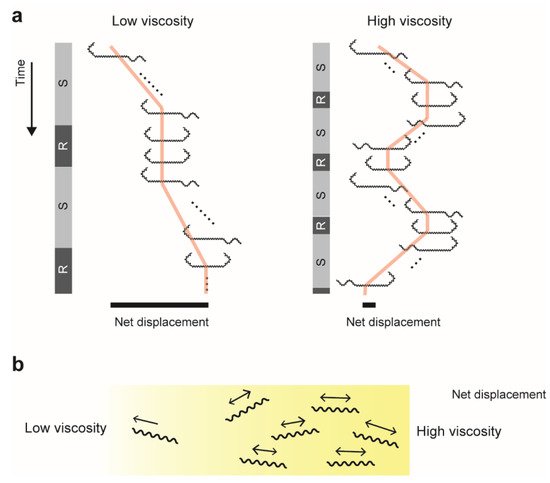
4. Swimming in a Highly Viscous Milieu
4.1. Dependence on a Type of Polymer
4.2. Back-and-Forth Motion
4.3. Trial and Error?
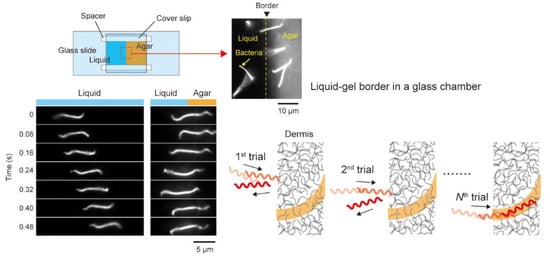
4.4. Interaction between PFs
This entry is adapted from the peer-reviewed paper 10.3390/ijms23031859
References
- Miyata, M.; Robinson, R.C.; Uyeda, T.Q.P.; Fukumori, Y.; Fukushima, S.; Haruta, S.; Homma, M.; Inaba, K.; Ito, M.; Kaito, C.; et al. Tree of Motility—A Proposed History of Motility Systems in the Tree of Life. Genes Cells 2020, 25, 6–21.
- Jarrell, K.F.; McBride, M.J. The Surprisingly Diverse Ways That Prokaryotes Move. Nat. Rev. Microbiol. 2008, 6, 466–476.
- Nakamura, S.; Minamino, T. Flagella-Driven Motility of Bacteria. Biomolecules 2019, 9, 279.
- Miyata, M. Unique Centipede Mechanism of Mycoplasma Gliding. Annu. Rev. Microbiol. 2010, 64, 519–537.
- Faure, L.M.; Fiche, J.-B.; Espinosa, L.; Ducret, A.; Anantharaman, V.; Luciano, J.; Lhospice, S.; Islam, S.T.; Tréguier, J.; Sotes, M.; et al. The Mechanism of Force Transmission at Bacterial Focal Adhesion Complexes. Nature 2016, 539, 530–535.
- Burrows, L.L. Pseudomonas aeruginosa Twitching Motility: Type IV Pili in Action. Annu. Rev. Microbiol. 2012, 66, 493–520.
- Wilde, A.; Mullineaux, C.W. Motility in Cyanobacteria: Polysaccharide Tracks and Type IV Pilus Motors. Mol. Microbiol. 2015, 98, 998–1001.
- Haiko, J.; Westerlund-Wikström, B. The Role of the Bacterial Flagellum in Adhesion and Virulence. Biology 2013, 2, 1242–1267.
- Josenhans, C.; Suerbaum, S. The Role of Motility as a Virulence Factor in Bacteria. Int. J. Med. Microbiol. 2002, 291, 605–614.
- Adler, B.; de la Peña Moctezuma, A. Leptospira and Leptospirosis. Vet. Microbiol. 2010, 140, 287–296.
- Picardeau, M. Virulence of the Zoonotic Agent of Leptospirosis: Still Terra Incognita? Nat. Rev. Microbiol. 2017, 15, 297–307.
- Coburn, J.; Picardeau, M.; Woods, C.W.; Veldman, T.; Haake, D.A. Pathogenesis Insights from an Ancient and Ubiquitous Spirochete. PLoS Pathog. 2021, 17, e1009836.
- Lambert, A.; Picardeau, M.; Haake, D.A.; Sermswan, R.W.; Srikram, A.; Adler, B.; Murray, G.A. FlaA Proteins in Leptospira Interrogans Are Essential for Motility and Virulence but Are Not Required for Formation of the Flagellum Sheath. Infect. Immun. 2012, 80, 2019–2025.
- Wunder, E.A.; Figueira, C.P.; Benaroudj, N.; Hu, B.; Tong, B.A.; Trajtenberg, F.; Liu, J.; Reis, M.G.; Charon, N.W.; Buschiazzo, A.; et al. A Novel Flagellar Sheath Protein, FcpA, Determines Filament Coiling, Translational Motility and Virulence for the Leptospira Spirochete. Mol. Microbiol. 2016, 101, 457–470.
- Goldstein, S.F.; Charon, N.W. Multiple-Exposure Photographic Analysis of a Motile Spirochete. Proc. Natl. Acad. Sci. USA 1990, 87, 4895–4899.
- Takabe, K.; Tahara, H.; Islam, M.S.; Affroze, S.; Kudo, S.; Nakamura, S. Viscosity-Dependent Variations in the Cell Shape and Swimming Manner of Leptospira. Microbiology 2017, 163, 153–160.
- Nakamura, S.; Leshansky, A.; Magariyama, Y.; Namba, K.; Kudo, S. Direct Measurement of Helical Cell Motion of the Spirochete Leptospira. Biophys. J. 2014, 106, 47–54.
- Tahara, H.; Takabe, K.; Sasaki, Y.; Kasuga, K.; Kawamoto, A.; Koizumi, N.; Nakamura, S. The Mechanism of Two-Phase Motility in the Spirochete Leptospira: Swimming and Crawling. Sci. Adv. 2018, 4, eaar7975.
- Takabe, K.; Nakamura, S.; Ashihara, M.; Kudo, S. Effect of Osmolarity and Viscosity on the Motility of Pathogenic and Saprophytic Leptospira. Microbiol. Immunol. 2013, 57, 236–239.
- Abe, K.; Kuribayashi, T.; Takabe, K.; Nakamura, S. Implications of Back-and-Forth Motion and Powerful Propulsion for Spirochetal Invasion. Sci. Rep. 2020, 10, 13937.
- Cox, P.J.; Twigg, G.I. Leptospiral Motility. Nature 1974, 250, 260–261.
- Charon, N.W.; Lawrence, C.W.; O’Brien, S. Movement of Antibody-Coated Latex Beads Attached to the Spirochete Leptospira Interrogans. Proc. Natl. Acad. Sci. USA 1981, 78, 7166–7170.
- Schneider, W.R.; Doetsch, R.N. Effect of Viscosity on Bacterial Motility. J. Bacteriol. 1974, 117, 696–701.
- Berg, H.C.; Turner, L. Movement of Microorganisms in Viscous Environments. Nature 1979, 278, 349–351.
- Magariyama, Y.; Kudo, S. A Mathematical Explanation of an Increase in Bacterial Swimming Speed with Viscosity in Linear-Polymer Solutions. Biophys. J. 2002, 83, 733–739.
- Nakamura, S.; Adachi, Y.; Goto, T.; Magariyama, Y. Improvement in Motion Efficiency of the Spirochete Brachyspira Pilosicoli in Viscous Environments. Biophys. J. 2006, 90, 3019–3026.
- Ruby, J.D.; Charon, N.W. Effect of Temperature and Viscosity on the Motility of the Spirochete Treponema Denticola. FEMS Microbiol. Lett. 1998, 169, 251–254.
- Kimsey, R.B.; Spielman, A. Motility of Lyme Disease Spirochetes in Fluids as Viscous as the Extracellular Matrix. J. Infect. Dis. 1990, 162, 1205–1208.
- Kaiser, G.E.; Doetsch, R.N. Enhanced Translational Motion of Leptospira in Viscous Environments. Nature 1975, 255, 656–657.
- Terasawa, S.; Fukuoka, H.; Inoue, Y.; Sagawa, T.; Takahashi, H.; Ishijima, A. Coordinated Reversal of Flagellar Motors on a Single Escherichia Coli Cell. Biophys. J. 2011, 100, 2193–2200.
- Charon, N.W.; Cockburn, A.; Li, C.; Liu, J.; Miller, K.A.; Miller, M.R.; Motaleb, M.A.; Wolgemuth, C.W. The Unique Paradigm of Spirochete Motility and Chemotaxis. Annu. Rev. Microbiol. 2012, 66, 349–370.
- Vig, D.K.; Wolgemuth, C.W. Swimming Dynamics of the Lyme Disease Spirochete. Phys. Rev. Lett. 2012, 109, 218104.
- Takabe, K.; Kawamoto, A.; Tahara, H.; Kudo, S.; Nakamura, S. Implications of Coordinated Cell-Body Rotations for Leptospira Motility. Biochem. Biophys. Res. Commun. 2017, 491, 1040–1046.
