Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cardiac & Cardiovascular Systems
Cardiovascular diseases (CVD) have a lower prevalence in women than men; although, a higher mortality rate and a poorer prognosis are more common in women. However, there is a misperception of CVD female risk since women have commonly been considered more protected so that the real threat is vastly underestimated. Consequently, female patients are more likely to be treated less aggressively, and a lower rate of diagnostic and interventional procedures is performed in women than in men. In addition, there are substantial sex differences in CVD, so different strategies are needed.
- cardiovascular disease
- women
- gender
- cardiovascular risk factors
. Introduction
Despite a lower prevalence of cardiovascular diseases (CVD) in women than men, the mortality rate and prognosis are poorer in females [1]. Women have been conventionally considered more protected, and, therefore, their real CVD risk has been largely underestimated [2,3,4,5]. As a result, less aggressive strategies are more likely to be used in women than men [2,4,5,6], as demonstrated by the lower rate of diagnostic and interventional procedures performed in females [2,4,6]. In addition, women are generally under-represented in most clinical trials [4,7]. Gender-related disparities in heart physiology have been widely demonstrated, leading to sex differences in CVD, which significantly influence different treatment strategies [4,8,9].
Therefore, CVD management should have a gender-specific approach that remains poorly applied in clinical practice. This study aimed to review main cardiovascular (CV) risk factors in women related to CVD and to discuss sex-specific treatment aiming at helping clinicians in adopting a more gender-specific clinical approach.
2. Cardiovascular Risk Factors in Women
The classic risk factors for CVD are comparable in women and men, but gender differences in the prevalence of each risk factor and unique factors exist for women (Figure 1). Indeed, smoking and dyslipidemia are more prevalent among men, whereas metabolic syndrome, sedentary, concomitant autoimmune, and chronic kidney diseases (CKD) are more frequent in women [5].
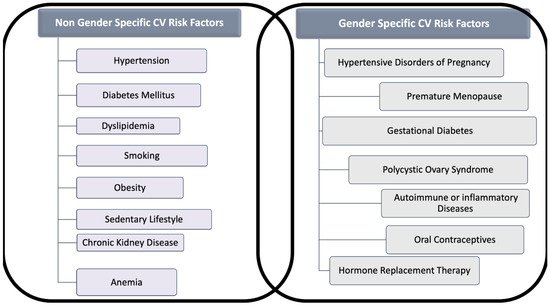
Figure 1. Gender and Non-Gender Cardiovascular (CV) Risk factors for cardiovascular disease in women. The figure distinguishes risk factors for cardiovascular disease in two categories: (A) Those that are related to gender, often under-recognized and (B) those that interest both sexes, but which might act in women differently than in men.
In Europe, data from the EUROASPIRE IV, a multi-centric study involving 7998 patients (24.4% females) referred to 78 centers in 24 countries for coronary heart disease (CAD), evidenced a poor risk factor management in coronary heart disease (CAD) in women than men [10,11].
According to these findings, the latest EUROASPIRE V survey (undertaken on 8261 CAD patients, 25.8% females) [12] showed a worse control of CV risk factors in women. On the contrary, a little gender gap in CV drugs intake has been evidenced [13].
Nevertheless, data analysis focused on gender differences in the patients’ awareness, showed a lower awareness about weight but a greater awareness about blood pressure (BP) and cholesterol target achievement in females [14].
2.1. Hypertension
Hypertension is the most usual modifiable risk factor for CVD, and lowering BP prevents morbidity and mortality in both sexes [15]. Premenopausal women usually have lower BP values than men [16]. However, after menopause, a steeper rise in hypertension rates is seen in women and about 80% of women aged ≥75 years have hypertension [17].
Females develop more often isolated systolic hypertension (ISH), reflecting aortic stiffness (AoS), and have a higher prevalence of strokes and heart failure (HF) with preserved ejection fraction (HFpEF) [18].
Hypertension is more frequently uncontrolled in women. Such types of hypertension are exclusive of women, such as hypertension related to oral contraceptive (COCs) use or hypertensive disorders during pregnancy (HPD) [19,20].
Several specific sex/gender factors could explain women’s unique arterial hypertension pathophysiology. Estrogens deficiency in post-menopause plays a crucial role in hypertension development due to adaptations of the sympathetic nervous system (SNS), renin-angiotensin-aldosterone system (RAAS), body mass (BM), endothelial function, oxidative stress, and salt sensitivity [20].
However, recent studies have also shown that differences in SNS, RAAS activation, sex chromosomes, and immune system, independently by the gonadal hormone status, contribute to the sexual dimorphism in BP control [21].
Nonetheless, there is currently no substantial evidence showing different efficacy of antihypertensive therapy based on gender.
In a large meta-analysis including 87,349 women, Turnbull et al. evaluated different BP-lowering regimens using similar cut-offs for men and women, showing equal protection against severe vascular complications in both sexes [22]. In this study, calcium channel blockers (CCBs) reduced the risk of stroke more than beta-blockers (BBs) or ACE inhibitors (ACEI) only in women, but not in men. However, CCBs did not differ from BBs, ACEI, or diuretics in protecting CAD, cardiac death, or death from any other cause in both genders.
Therefore, guidelines for managing arterial hypertension recommend no different BP targets or particular drug classes, based on the patient’s gender [23].
It’s well known that current guidelines suggest a more intensive treatment of hypertension to a goal systolic BP ≤ 130 mmHg, based on large trials such as SPRINT (Systolic Blood Pressure Intervention Trial). However, a prespecified subgroup analysis of this study failed to show a statistically significant benefit from the intensive treatment versus the standard therapy in women [24].
Further studies, including larger women population with hypertension, are needed to test the hypothesis for implementing more gender-specific treatment indications.
Therefore, to date, gender should not influence selecting antihypertensive therapies, apart from evaluating gender-specific side effects or contraindications in pregnancy [20].
Common side effects of antihypertensive therapy occur more frequently among women than men. ACEI-induced cough is twice as common in women than in men, and women are more likely to complain of CCBs related peripheral edema and to develop diuretic-induced hyponatremia and hypokalemia [20,25]. On the other hand, diuretics might positively affect the prevention of osteoporosis in postmenopausal women through reduced urinary calcium excretion [24,25].
Gender Differences in Hypertension-Related Target Organ Damage
It is well known that hypertension-mediated organ damage (HMOD) in vessels or organs (heart, brain, eyes, and kidney) is a marker of pre-clinical CVD associated with increased CV morbidity and mortality [26]. Therefore, knowledge of the presence of HMOD is of significant importance to better stratify CV risk and for the optimal management of hypertensive patients [26]. Several gender differences in HMOD have been described in the last years, and estrogens play a crucial role in HMOD pathogenesis [20].
Postmenopausal hypertensive women have more often ISH, reflecting an increase in AoS [27], more concentric left ventricular (LV) remodeling and less LV in response to arterial hypertension, resulting in a higher LV mass index and greater prevalence of HFpEF [18]. Moreover, in women, it has been demonstrated that the regression of hypertensive left ventricular hypertrophy (LVH) is more difficult to be obtained than in men, and residual hypertrophy is more common despite effective antihypertensive strategies and adequate BP control [28].
It is important to note that LVH has a well-demonstrated association with CV morbidity and mortality, and some studies have demonstrated that a higher LV mass index have a more significant impact on worse clinical outcome in women than in men [28].
Obesity, more prevalent in women than in men, may also potentiate the effect of hypertension on LVH in women, and the presence of increased body mass index (BMI) may be responsible, at least in part, for the lack of LVH regression as observed in the Strong Heart Study population [28,29].
Significant differences among male and female individuals were observed on vasculature damage, including arterial stiffness and intimate-medium thickness (IMT), carotid plaque size and compositions, and small arteries. Recently, extensive prospective studies showed that men have higher carotid IMT and carotid plaques than women at any decade of age. In contrast, women have less plaque burden, more stenosis, and a more positive remodeling of internal carotid arteries [30]. Intraplaque hemorrhage, a marker of plaque instability, is more frequent in men than women. Still, with increasing age, the probabilities of intraplaque carotid bleeding in women become closer to that of men [31]. It has been well assessed that arterial stiffness increases more significantly in women with aging, related to two-fold higher mortality than men [27].
Coronary microvascular dysfunction (CMD) leads to a significant increase in endothelial shear stress which negatively influences coronary anatomy and function and is strongly correlated with adverse CV events [32,33]. Moreover, the smaller coronary arteries size associated with a higher blood flow has been reported as a causal factor of a greater prevalence of CMD in women. In addition, the direct effect exerted by hypertension on microcirculation causes intramural arterioles’ remodeling and interstitial fibrosis. The reduction in microvascular density has also been involved in the development of CMD [32]. Nevertheless, estrogens have a protective role in premenopausal women [34]. The mechanism would seem due to early estrogen loss resulting in chronic activation of the RAAS [20]. Therefore the incidence of CMD significantly rises in postmenopausal women [32].
A more significant and earlier hypertension-related microvascular dysfunction in the female sex has been recently supported by the findings that the media/lumen (ML) ratio was higher in women than in men after correction for classical CV risk factors and age [35].
In contrast, microvascular obstruction areas (also knowns as “no-reflow”) following myocardial infarction (MI) remodeling are smaller in women and more presumably linked to distal atherothrombotic embolization, microvascular impairment, and reperfusion insult [36].
Microalbuminuria is a marker of CV and renal diseases, and it is a sign of HMOD in essential hypertension [37]. Irrespective of BP levels, microalbuminuria, urinary creatinine, and albumin excretion is lower in women [38]. Postmenopausal women have a more rapid deterioration of renal function, while BP control results in higher proteinuria lowering men than in women.
Experimental animal studies suggest a role for T regulatory cells and RAS system in sex differences in hypertensive kidney injury [39].
Hypertensive retinopathy (HR), which refers to retinal microvascular signs which develop in response to elevated BP, predicts stroke, congestive heart failure (CHF), and CV mortality, independently of traditional risk factors [40]. Hypertensive retinal vascular signs can be classified into arteriolar changes (narrowing of the retinal arteriolar vessels due to vasospasm and increased vascular tone, arterio-venous crossing or nicking, and arteriolar wall opacification), and more advanced retinal lesions (microaneurysms, retinal hemorrhages, cotton-wool spots, hard exudates, optic nerve ischemia, and optic disk swelling) [41].
HR is more prevalent in males than in women. This difference may be explained by differential distribution in risk factors [42]. It has been shown that antihypertensive therapy results in regression of HR and that this effect is mainly due to BP reduction and rather than antihypertensive drugs [43].
2.2. Diabetes Mellitus
Diabetes mellitus (DM) is estimated to affect over 13 million women in the United States, with 90–95% having type 2 diabetes (T2DM) [43].
It has been observed that people with T2DM have a 2–3 times higher CV risk than people without diabetes [44]. Therefore preventing microvascular complications could reduce major adverse CV events, as far as T2DM, is involved in CAD development, in plaque burden, in the lesion extent and vascular remodeling, hesitating in a severe and diffuse coronary artery narrowing [45,46].
Regarding females, it has been assessed that diabetes significantly attenuates premenopausal cardioprotection [47]. Women with T2DM rise a greater CV risk compared with non-diabetic women and diabetic men both [47]. A greater risk of CVD mortality in diabetic women compared with men has also been reported [47]. A more pronounced hypercoagulability, endothelium dysfunction, and metabolic and cellular alterations resulting in functional and structural abnormalities are involved in the mechanism of myocardial dysfunction with a poor impact in women [43]. In addition, an enhanced risk of HF as well as HF mortality has been long been recognized in women with T2DM compared with men [47] (Figure 2).
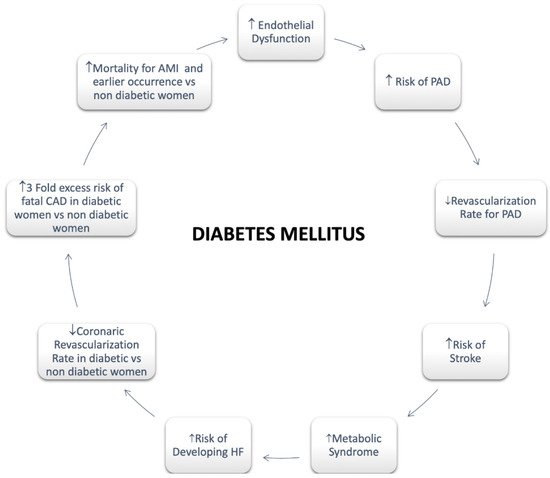
Figure 2. Diabetes Mellitus in Women. Abbreviations: Peripheral artery disease; HF: Heart Failure; CAD: Coronary Artery Disease; AMI: Acute Myocardial Infarction.
The T2DM pathogenesis is strictly linked to obesity. Thus, it is known that the BMI, adipose tissue dysfunction and the expression of adipokines secreted by the adipose tissue play an essential role in the T2DM etiopathogenesis [29,48]. Since all these features significantly differ between men and women, sex differences are particularly relevant in T2DM [48,49]. To be more specific, obesity is more prevalent in women [50], whereas in men there is a higher risk of developing T2DM [51]. However, accumulating visceral fat is linked to the development of T2DM [52].
Moreover, insulin sensitivity is more frequently observed in women [53]. However, the progressive loss of estrogen production during aging slowly results in significant changes in body shape, increasing abdominal fat storing, and shifting from the gynoid to the android shape [54]. Finally, CV relative risk seems to be more strongly correlated to T2DM in women [55].
After these considerations, we may state that an aggressive approach for diabetic patients is required in both sexes. A lighter treatment based on their hypothetic, more favorable hormones profile is no longer acceptable [55].
2.3. Cholesterol
A higher prevalence of elevated total cholesterol (TC) levels and lower high-density lipoprotein cholesterol (HDL-C) values have been shown in women than in men [56]. In Italy, in 2008–2012, the levels of total TC and low-density lipoprotein cholesterol (LDL-C) were lower in men, with a prevalence of 65% of TC > 200 mg\dL, compared to the 69% of women [57].
The 2018 ACC/AHA cholesterol guidelines identified during early menopause a rise in LDL and total cholesterol with increased CV risk [19].
Although a strong correlation between menopause and changes in cholesterol levels has been previously described, a more precise assessment of the significant increase in TC, LDL-C, and Apolipoprotein B (ApoB) levels occurring in the final menstrual period (FMP) has been well recognized in SWAN study(Study of Women’s Health Across the Nation) [58,59]. Additionally, relevant variations in carotid plaque burden have also been observed in the follow-up [59,60].
In the Tromso Study, an association between carotid atherosclerosis and earlier menopausal age was reported [61]. Finally, premenopausal values of LDL-C, HDL-C, and triglycerides have been well identified as strong predictors of carotid IMT in the postmenopausal phase in the Pittsburgh Healthy Women Study [62].
Therefore, the phase between one year before and one year after FMP should be considered as the critical time for lipid profile changes. As a consequence, a more careful lipid monitoring approach in premenopausal and perimenopausal women should be performed [47].
The INTERHEART TC/HDL-C. The INTERHEART study has investigated the Apolipoprotein B (ApoB)/ Apolipoprotein A1 (ApoA1) and TC/HDL-C ratios finding an association with acute myocardial infarction (AMI) more frequently in women than in men [63].
Statins have an indication in secondary prevention without difference of gender for major CV events [64]. In recent years, the aggressive reduction of LDL cholesterol with the Proprotein Convertase Subtilisin/Kexin type 9 (PCSK9 inhibitors) contributed to the significant reduction in ischemic events without apparent gender differences [65].
2.4. Smoking
A recent World Health Organization (WHO) report on the global tobacco epidemic showed that, in 2013, 19% of women and 38% of men aged 15 years old and above smoked tobacco in the WHO European Region. This average among European women is sensibly higher than those observed in the WHO African, South-East Asia, Eastern Mediterranean and Western Pacific Regions (2–3%) [65]. The INTERHEART study reported that smoking had a similar risk of AMI in both genders [66]. An increase in tobacco or e-cigarette smoking has been documented in the last years, contributing to a 25% increase in CV risk [67].
Consequently, the prevalence of women smokers is becoming higher than men, impacting morbidity and mortality connected to smoking-related diseases [68].
2.5. Obesity
Obesity rates are rising worldwide, involving about a third of the world’s population [69].
In the WHO European Region, the age-standardized prevalence of obesity in 2016 was 21.85% for men and 24.46% for females, with an increasing parallel trend in two genders [68].
A higher prevalence of obesity in women (18%) compared to men (10%) occurs [70]. Moreover, obesity in pregnancy may contribute to the development of hypertension and GD [71].
BMI is commonly used to define overweight or obese patients, although either important is the fat localization [72]. According to data from the Framingham Heart Study, the excess risk of cardiovascular disease CVD attributed to obesity after adjustment for waist circumference was 64% in women versus 46% in men [73]. In a study by Chen et al. including 2863 postmenopausal women, trunk fat was strongly associated with CV risk despite a normal BMI [74]. Central obesity is more common in women than men contributing to metabolic syndrome (MS), especially in postmenopausal women [75] (Figure 3). According to data from the Framingham Heart Study, the excess risk of CVD attributed to obesity after adjustment for waist circumference was 64% in women versus 46% in men [73].
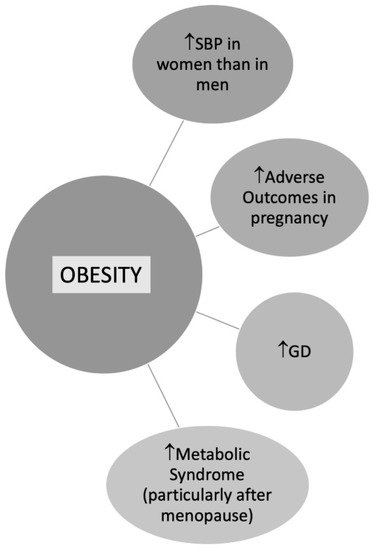
Figure 3. Obesity in Women. Abbreviations: SBP: Systolic blood pressure; GD: Gestational diabetes.
2.6. Physical Activity
By the pooled data from 358 surveys on physical activity across 168 countries, including 1.9 million population between 2001 and 2016, authors reported a higher rate of physical inactivity in women than men (31.7% versus 23.4% in 2016) [76].Young women are less engaged in physical activity (PA) than men, with a continuing decrease over the years leading to increased risk of CV disease [43,77]. Extending the duration of physical activity beyond 10 minutes in older people is essential for staying healthy [78].
2.7. Chronic Kidney Disease
In addition to the conventional CV risk factors, CKD is strongly associated with CV events [79]. An enhanced prevalence of CKD in women, including primary injuries and secondary involvements in systemic diseases, has already been described [80,81] Women who have a longer life expectancy because of their age, have a greater reduction in the glomerular filtrate rate (GRF), which could be a potential cause of the more CKDs prevalence in females. Additionally, it has been hypothesized that a significant role of sex hormones is involved in gender disparities [82].
Furthermore, it has been shown that women are more likely to be affected by autoimmune diseases like Systemic Lupus Erythematosus (SLE) occurring in their childhood [83,84]. Therefore, SLE-related nephritis (Lupus Nephritis, LN), has been reported in more than 75% of SLE patients [85]. LN, resulting from autoimmune mechanisms, may lead to kidney failure [84]. Furthermore, Systemic Sclerosis (SS), prevalently affecting women, could determine a kidney impairment in 5% of patients [86]. In addition, pyelonephritis, more common in women for anatomic features, can lead to CKD over time [87].
Moreover, a significant relationship has been found between pregnancy and CKD. It seems to be related to complex anatomic-functional modifications of kidneys that characterize maternal physiopathology. Both Acute Kidney Injury (AKI) and preeclampsia (PE) can be pregnancy-related complications leading to the development of CKD [88]. AKI represents a preeminent problem significantly increasing maternal and fetal morbidity and mortality [88]. Moreover, AKI often results in CKD and end-stage kidney disease (ESKD). Consequently, prompt recognition and suitable treatment for AKI are mandatory during pregnancy [88].
Preeclampsia occurs in 5–8% of pregnancies causing 15–20% of pregnancy-related AKI representing a potential cause of intrauterine and perinatal mortality, preterm delivery, and intrauterine growth restriction (IUGR) [88].
2.8. Anemia
Anemia is largely diffused among the general population. It is significantly influenced by economic status and consequently by nutritional deficiencies representing a worldwide health problem. Despite its gender-balanced spread, anemia is particularly common in women [91], with a prevalence of 38% during pregnancy and 29% in non-pregnant women [92].
Iron deficiency (ID) is the preeminent cause of anemia in females with an incidence ranging between 15 to 18% [91]. The etiology of ID in women is multifactorial [93]. Slow bleeding from uterine fibroids, heavy menstruation [94], intrauterine devices (IUDs), and other gynecological conditions have been considered as causative factors [92,95]. Moreover, hemoglobinopathies, gastrointestinal (GI) bleedings, autoimmune diseases, kidney failure, parasitosis, other nutritional deficiencies (such as vitamin B12, folate), acute and chronic diseases, and malabsorption are the other most common general causes of anemia [92,93,96].
The WHO established that a reduction of 50% of anemia in fertile females is part of the six global nutritional goals to be achieved by 2025 [97], setting the cut-off hemoglobin (Hb) concentration at <110 gl and <120 gl for pregnant women and non-pregnant women, respectively [98]. Vegetarian or vegan diets [99], younger and older maternal age [100], multiple pregnancies [101], and previous anemias are predisposing risk factors of developing ID in pregnancy.
If anemia has been confirmed and other causes of bleeding have been excluded, gastroscopy and colonoscopy should be recommended [102]. Finally, a higher need for transfusion after surgery has been reported in women undergoing surgery [91]. Oral or intravenous iron supplements are the recommended treatment after the exclusion of removable causes [91].
This entry is adapted from the peer-reviewed paper 10.3390/jcm11051176
This entry is offline, you can click here to edit this entry!
