1. The Wiring of the Mammalian Retina
The mammalian retina is a valuable and common model in neuroscience, due to its well-defined layered structure and its excellent potential for conducting in vitro electrophysiological studies. This has allowed scientists to map many of the neuronal connections and build a wiring blueprint (
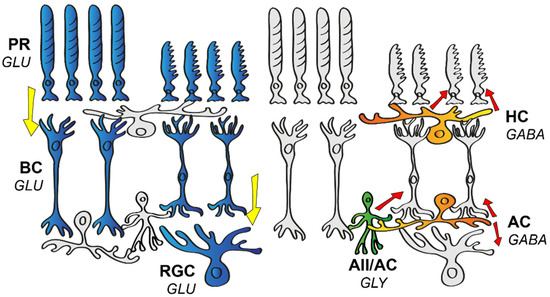
Figure 1) that brings functional and morphological connections in the retina together in one schematic [
1]. Elements of this blueprint are generally found in all mammalian species, including both primate and non-primate models and humans as well. The researchers' current knowledge regarding neurons in the mouse retina points to at least 130 neuronal cell types, with 2 distinct photoreceptor types (PRs) [
2], 1 type of horizontal cell (HC) [
3], 15 different bipolar cell types (BCs) [
4], roughly 63 diverse amacrine cell types (ACs) [
5], and an estimated number of around 46 retinal ganglion cell types (RGCs) [
6]. Just like in any other species, visual perception in mice begins at the level of PRs, where rods and cones create two separate pathways for daytime (high intensity or photopic) or nighttime (low intensity or scotopic) illumination, respectively. Rods are sensitive enough to detect a single photon [
7], and thus they are most efficiently utilized in low-light environments. Cones, on the other hand, express photopigments that are less sensitive to the intensity of the stimulating light, but the variety in the expressed chromophores allows them to specialize to a certain wavelength, which has proven to be the first step in establishing color vision. The human retina contains 3 cone subtypes specifically adapted to peak sensitivity at short (S, ~430 nm), medium (M, ~530 nm), and long (L, ~560) wavelengths [
8], while the mouse retina only possesses S and M sensitive cones [
2].
Figure 1. Schematic drawing of the basic neuronal wiring of the mammalian retina. The vertical information stream encompasses the light-sensitive photoreceptors (PR), bipolar cells (BC), and retinal ganglion cells (RGC). These cells express glutamate (GLU), thus providing an excitatory (highlighted in blue) stream of signals (direction of information flow is indicated by yellow arrows). Horizontal cells (HC) and amacrine cells (AC) serve various forms of inhibition (red arrows) in the outer or the inner retina, respectively. HCs express GABA (highlighted in orange in the outer retina), whereas ACs release either glycine (GLY, highlighted in green) or GABA (highlighted in orange in the inner retina) as a neurotransmitter.
PRs form synapses with 15 different types of BCs, with one BC collecting inputs from about 5–10 presynaptic PRs [
9]. BCs lay the foundation for a number of parallel signaling pathways at their level [
10] and the segregation of information is maintained throughout the downstream circuitry to higher visual brain centers. Roughly half of these parallel pathways inform the brain about an increase in light intensity (ON pathways), whereas the other half signals decrements in light intensity (OFF pathways). This ON and OFF polarity distinction appears to be so fundamental for vision that ON and OFF signals are established as early as the first retinal synapse (the PR-BC synapse). BCs that express ionotropic glutamate receptors (BCs: 1(ab), 2, 3(a,b), 4) are responsible for forming the OFF signaling routes [
11,
12], whereas those that utilize metabotropic glutamate receptors (5(a–d), 6, 7, 8/9) are responsible for conveying ON signals [
11]. The first synaptic layer of the retina is also equipped with HCs that express inhibitory GABA as a transmitter and signal laterally, thereby providing feedback and feedforward inhibition to PRs and BCs, respectively [
13]. This inhibition serves mainly as a spatial filter and thus forms the basis for an inhibitory surround receptive field of downstream retinal neurons and thus contrast detection for vision [
14].
ON and OFF BCs both release glutamate and synapse with ON, OFF, or ON–OFF RGCs and a large variety of ACs. In turn, BCs receive AC inhibitory inputs at their axon terminals through reciprocal feedback and/or feedforward mechanisms [
15]. ACs are commonly classified into glycinergic narrow or GABAergic wide-field populations [
16]. ACs in general exhibit great diversity in terms of morphology and function, and according to a recent study, their molecular makeup indicates as many as 63 separate subtypes [
5]. While narrow-field ACs modulate local glutamate input of BCs, wide-field ACs generally provide a non-linear surround to RGCs [
17]. Through gap junction (GJ) coupling, ACs can also relay excitatory input to neighboring ACs or even to neurons of other cell types. One of the best-known examples of ACs with GJ coupling are the narrow-field AII ACs that, upon receiving excitatory glutamate input from rod BCs [
18], use gap junctions to excite ON cone BCs, and glycinergic chemical synapses to inhibit OFF BCs at the same time [
19]. ACs might also provide a sign-inverting feedforward mechanism, such as in the case of S-M (short wavelength–medium wavelength) color opponency in the murine retina, where an S-ON/M-OFF RGC will receive direct excitatory input from S-ON BCs and inhibition through a sign-inverting AC connection from an M-ON BC [
20]. Interestingly, there is at least one AC type (VGlut3 expressing AC) that utilizes a glutamatergic excitatory signaling mechanism to provide a feedforward (ON to ON, OFF to OFF) and crossover (ON to OFF, OFF to ON) glutamatergic input in the case of direction-selective ganglion cells (DSGCs) [
21]. Once visual signals have passed through all the above-mentioned circuit elements, they are integrated by RGCs to forge spike trains that inform the brain about the change in only one specific visual aspect [
22].
2. RGC Response Transience and Possible Visual Functions
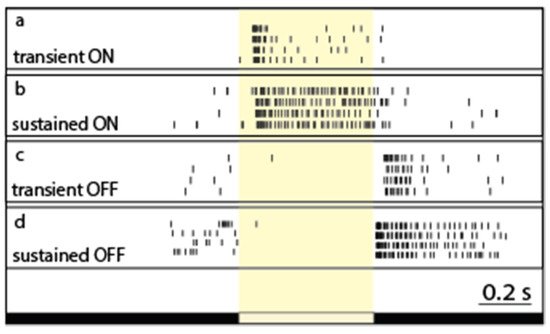
Light-evoked RGC responses have been characterized by their polarity (ON, OFF, and ON–OFF), sensitivity to various stimuli, and their kinetics. In general, the two main response parameters that are considered and studied when it comes to RGC response kinetics are the response delay and the response decay. The response delay reflects the speed by which a certain neuron reacts to an incoming stimulus, and thus neurons including RGCs are generally considered either brisk or sluggish. On the other hand, response decay reveals the ability of a neuron to maintain its activity without inactivation in response to continuous stimulation. Based on different manifestations of the latter phenomenon, neurons are known to display either maintained spiking activity (sustained response) or present a brisk spike burst (transient response) in response to continuous stimulation (
Figure 2). Both aspects (response delay and decay) likely contribute to signal efficiency on postsynaptic neuronal targets in higher visual centers [
23,
24,
25]. The transient/sustained dichotomy of RGC response decay has been documented in a variety of vertebrate species, including cold-blooded animals, primates, and non-primate mammals as well [
26,
27,
28,
29,
30,
31,
32,
33,
34,
35,
36], indicating that this kinetic feature has been conserved throughout vertebrate evolution and thus further attesting the importance of the role that RGC response kinetics play in visual coding.
Figure 2. The diversity of response transience across the retinal ganglion cell population. Peri-event rasters of representative RGCs (a–d). Light-evoked spiking responses upon full-field illumination are rather similar across trials for each RGC, but they display a great variety in terms of their response length (or decay—expressed as the PSTHτ value in this work) for both the ON (cells 1 and 2) and OFF (cells 3 and 4) subpopulations. The white bar below the recordings represents the timing of the on- and off-set of the stimulus.