Asthma is a respiratory condition often stemming from childhood, characterized by difficulty breathing and/or chest tightness. Current treatment options for both adults and children include beta-2 agonists, inhaled corticosteroids (ICS), and leukotriene modifiers (LTM). Despite recommendations by the Global Initiative for Asthma, a substantial number of patients are unresponsive to treatment and unable to control symptoms. Pharmacogenomics have increasingly become the front line of precision medicine, especially with the recent use of candidate gene and genome- wide association studies (GWAS). Screening patients preemptively could likely decrease adverse events and therapeutic failure. However, research in asthma, specifically in pediatrics, has been low. Although numerous adult trials have evaluated the impact of pharmacogenomics and treatment response, the lack of evidence in children has hindered progress towards clinical application.
- pediatric asthma
- pharmacogenomics
- beta-2 receptors
- inhaled corticosteroids
- leukotriene modifiers
1. Introduction
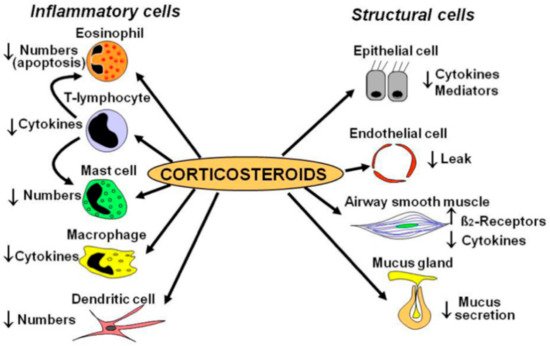
2. Inhaled Corticosteroids (ICS)
| Study | Population | Gene | Medication | Measurement | Results |
|---|---|---|---|---|---|
| Tantisira [51] | White | GLCCI1 | Budesonide | Change in FEV1 from baseline | rs37972 TT and rs37973 G allele variants showed unfavorable responses |
| Thompson [52] | European | GLCCI1 | ICS | Exacerbation (hospital visits and oral steroid use) | rs37973 G allele showed unfavorable response |
| Vijverberg [53] | Europe | GLCCI1 | Budesonide | Exacerbation (emergency room visits, hospital visits, oral steroid use) | No association found at rs37972 |
| Huang [54] | Chinese | GLCCI1 | ICS | MMEF | rs37969, rs37972, and rs37973 produced favorable response. |
| Kim [55] | Korean | HDAC1 | ICS | % change in FEV1 pre- and post-bronchodilator | rs1741981 CT and TT produced favorable response |
| Tantisira [56] | Caucasians | CRHR1 | Budesonide | % change in FEV1 from baseline | rs242941T produced favorable response |
| Tantisira [57] | Multiethnic | TBX21 | Budesonide | % change in FEV1 and PC20 | rs2240017Q produced favorable response |
| Tantisira [58] | Multiethnic | FCER2 | Budesonide | Exacerbations (ER visits or hospitalization) | rs28364072 CC produced unfavorable response |
| Koster [59] | European | FCER2 | ICS, ICS + salmeterol, ICS + salmeterol + montelukast | Exacerbations (hospital visits and/or oral steroid use) | rs28364072 CC produced unfavorable response |
| Keskin [60] | Turkey | NR3C1 | Fluticasone | FEV1 improvement at 4 h | rs41423247 GG produced favorable response |
| Stockman [61] | Whites | CYP3A4, CYP3A5, and CYP3A7 | Fluticasone | Asthma control scores | CYP3A4 *22 produced favorable response |
| Stockman [62] | White | CYP3A4, CYP3A5, and CYP3A7 | Beclomethasone | Asthma control scores | CYP3A5 *3/*3 produced favorable response |
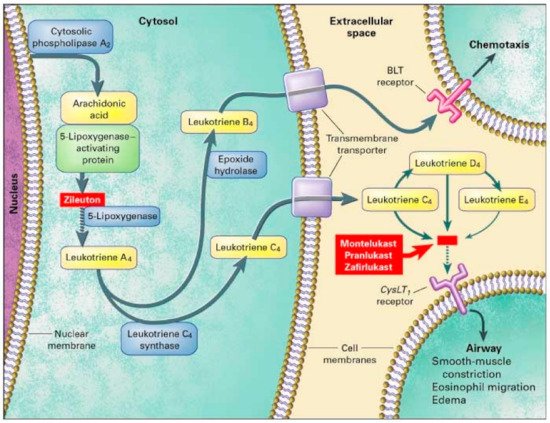
3. Leukotriene Modifiers (LTM)
3.1. Genes That Affect Montelukast Response
| Study | Population | Gene | Measurement | Results |
|---|---|---|---|---|
| Kim [67] | Korean | TBXA2 | FEV1 | Combination of +795 CT/CC and +924 TT showed unfavorable response |
| Klotsman [69] | Multiethnic | ALOX5 CystLTR2 LTC4S |
PEF PEF FEV1 and PEF |
rs4987105 TT and rs4986832 AA variants showed favorable outcomes rs912277 TT and rs912278 TC variants produced favorable response No association found at rs730012 |
| Telleria [70] | Spain | ALOX5 | % change in FEV1, exacerbations, and rescue inhaler need | rs59439148 5/5 and 5/4 copies showed favorable outcomes |
| Kang [71] | Korean | LTC4S PTGDR |
≥10% increase in FEV1 post exercise challenge | No significance at rs730012 rs803010 TT produced favorable response |
| Lee [72] | Korean | LTC4S CystLTR1 |
>10% increase in FEV1 post exercise challenge | No significance at SNP rs730012 No significance found |
| Whelan [73] | African American and Caucasian | LTC4S | FENO | rs730012 AC produced favorable response |
| Mougey [74] | Multiethnic | SLCO2B1 | Asthma symptom utility index (ASUI) | rs12422149 GG produced favorable response No association at rs2306168 |
| Li [75] | Chinese | SLCO2B1CYP2C8 | Drug clearance | Decreased clearance in rs12422149 GG No association found |
| Study | Population | Gene | Medication | Measurement | Results |
|---|---|---|---|---|---|
| Lipworth [47] | Scotland | ADRB2 | Fluticasone + montelukast or salmeterol + fluticasone | School absences, FEV1, asthma symptoms | Arg16Arg produced favorable responses to fluticasone and montelukast |
3.2. Genes Affecting Other Leukotriene Modifier Responses
| Study | Population | Gene | Medication | Measurement | Results |
|---|---|---|---|---|---|
| Tcheurekdjlan [79] | Mexican and Puerto Ricans | LTA4H ALOX5AP |
Albuterol + leukotriene modifiers (montelukast, zafirlukast, and zileuton) | % change in FEV1 pre and post bronchodilator | LTA4H rs2540491 A variants and rs2540487 GA variants produced favorable response No association of ALOX5AP variants alone rs10507391 A allele and rs9551963 C allele magnifies LTA4H response |
This entry is adapted from the peer-reviewed paper 10.3390/jor2010003
