Over the last three decades, proteins and peptides have attracted great interest as drugs of choice for combating a broad spectrum of diseases, including diabetes mellitus, cancer, and infectious and neurological diseases. However, the delivery of therapeutic proteins to target sites should take into account the obstacles and limitations related to their intrinsic sensitivity to different environmental conditions, fragile tertiary structures, and short half-life. Polymeric nanostructures have emerged as competent vehicles for protein delivery, as they are multifunctional and can be tailored according to their peculiarities.
- polymeric nanostructures
- therapeutic proteins
- protein delivery
- amphiphilic block copolymers
1. Introduction
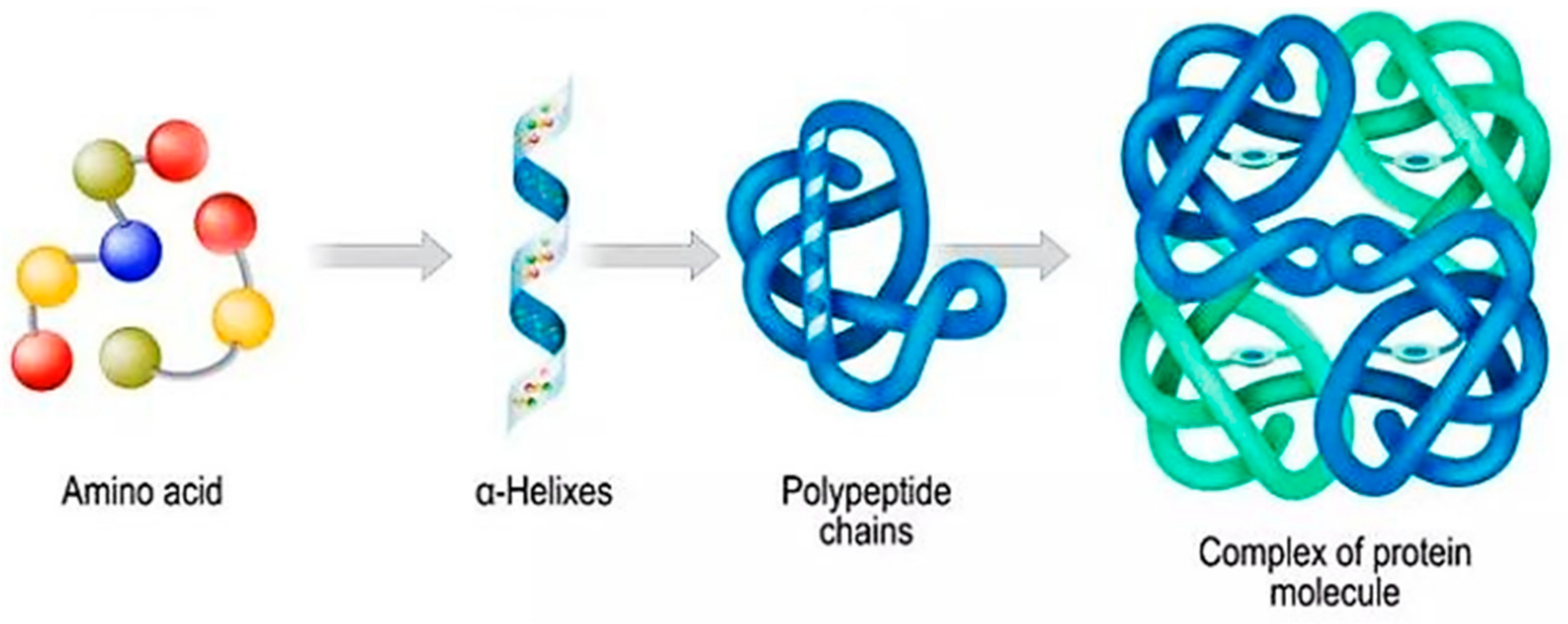
2. Polymeric Nanostructures
3. Linking Polymers, Proteins, and Peptides towards the Formation of Polymer–Protein–Peptide Nanostructures
3.1. Polymer–Protein Conjugation
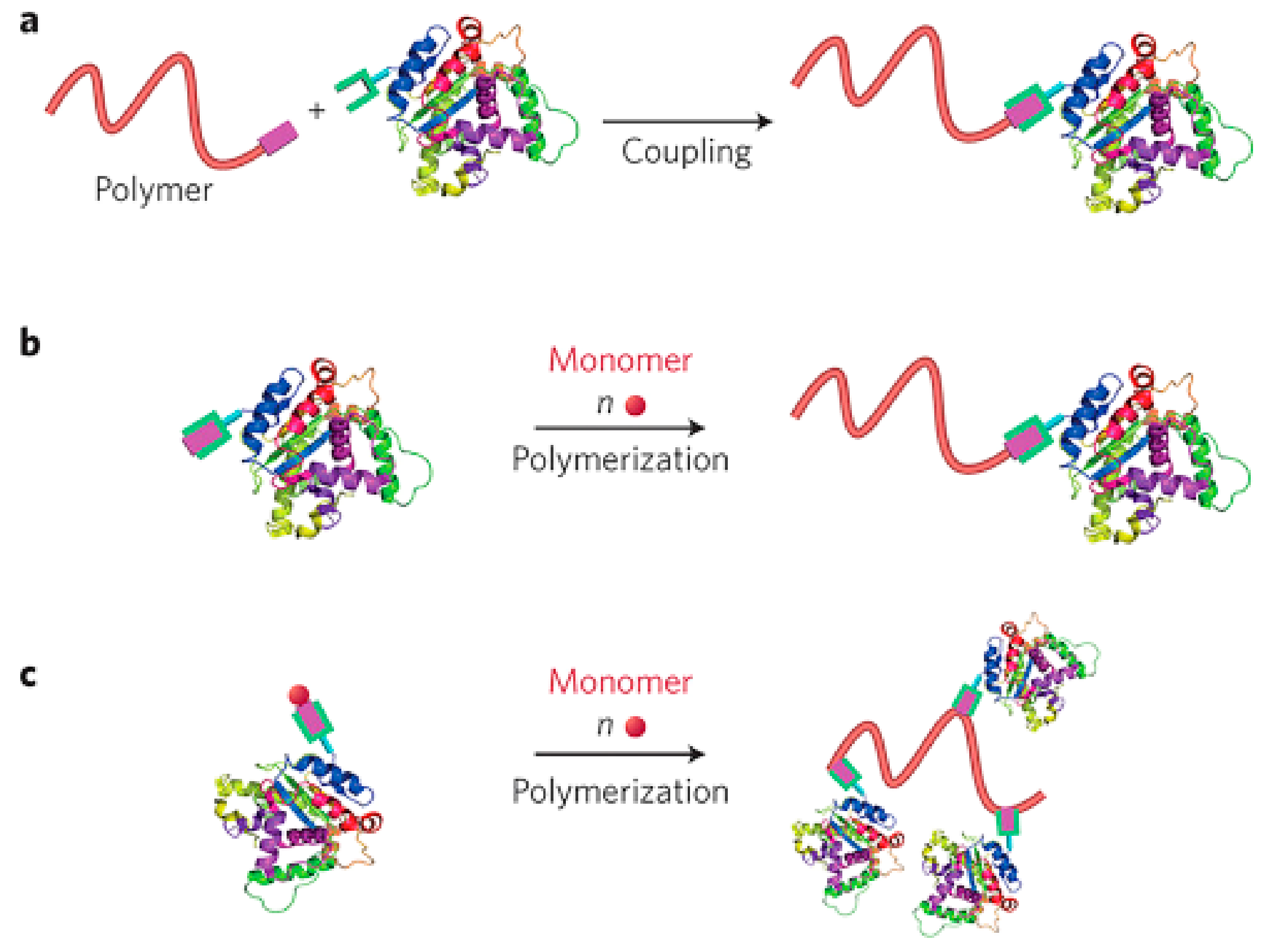
3.2. Chemical Bonding of Polymer Chains with Protein
3.3. Protein Encapsulation
This entry is adapted from the peer-reviewed paper 10.3390/polym14040777
References
- Ye, C.; Venkatraman, S. The long-term delivery of proteins and peptides using micro/nanoparticles: Overview and perspectives. Ther. Deliv. 2019, 10, 269–272.
- Chen, J.; Zou, Y.; Deng, C.; Meng, F.; Zhang, J.; Zhong, Z. Multifunctional Click Hyaluronic Acid Nanogels for Targeted Protein Delivery and Effective Cancer Treatment in Vivo. Chem. Mater. 2016, 28, 8792–8799.
- Al-Azzam, S.; Ding, Y.; Liu, J.; Pandya, P.; Ting, J.P.; Afshar, S. Peptides to combat viral infectious diseases. Peptides 2020, 134, 170402.
- Washburn, R.L.; Mueller, K.; Kaur, G.; Moreno, T.; Moustaid-Moussa, N.; Ramalingam, L.; Dufour, J.M. C-Peptide as a Therapy for Type 1 Diabetes Mellitus. Biomedicines 2021, 9, 270.
- Imran, M.; Shah, M.R.; Shafiullah. Chapter 10—Amphiphilic block copolymers–based micelles for drug delivery. In Design and Development of New Nanocarriers, 1st ed.; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 365–400.
- Walsh, G. Biopharmaceutical benchmarks 2018. Nat. Biotechnol. 2018, 36, 1136–1145.
- Deb, P.K.; Al-Attraqchi, O.; Chandrasekaran, B.; Paradkar, A.; Tekade, R.K. Chapter 16—Protein/Peptide Drug Delivery Systems: Practical Considerations in Pharmaceutical Product Development. In Basic Fundamentals of Drug Delivery, 1st ed.; Tekade, R.K., Ed.; Elsevier, Academic Press: Cambridge, MA, USA, 2019; pp. 651–684.
- Stratton, M.R.; Campbell, P.J.; Futreal, P.A. The cancer genome. Nature 2009, 458, 719–724.
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. 2015, 20, 122–128.
- Dastider, D.; Jyoti Sen, D.; Kumar Mandal, S.; Bose, S.; Ray, S.; Mahanti, B. Hand santizers bid farewell to germs on surface area of hands. Eur. J. Pharm. Sci. 2020, 7, 648–656.
- Lagassé, H.A.D.; Alexaki, A.; Simhadri, V.L.; Katagiri, N.H.; Jankowski, W.; Sauna, Z.E.; Kimchi-Sarfaty, C. Recent advances in (therapeutic protein) drug development. F1000Research 2017, 6, 113.
- Zhao, H.; Lin, Z.Y.; Yildirimer, L.; Dhinakar, A.; Zhao, X.; Wu, J. Polymer-based nanoparticles for protein delivery: Design, strategies and applications. J. Mater. Chem. B 2016, 4, 4060–4071.
- Hou, Y.; Lu, H. Protein PEPylation: A New Paradigm of Protein–Polymer Conjugation. Bioconjug. Chem. 2019, 30, 1604–1616.
- Srivastava, S.; Sharma, V.; Bhushan, B.; Malviya, R.; Awasthi, R.; Kulkarni, G.T. Nanocarriers for protein and peptide delivery: Recent advances and progress. J. Res. Pharm. 2021, 25, 99–116.
- Khodabakhsh, F.; Salimian, M.; Hedayati, M.H.; Ahangari Cohan, R.; Norouzian, D. Challenges and advancements in the pharmacokinetic enhancement of therapeutic proteins. Prep. Biochem. Biotechnol. 2021, 51, 519–529.
- Dellas, N.; Liu, J.; Botham, R.C.; Huisman, G.W. Adapting protein sequences for optimized therapeutic efficacy. Curr. Opin. Chem. Biol. 2021, 64, 38–47.
- Ding, S.; Zhang, N.; Lyu, Z.; Zhu, W.; Chang, Y.-C.; Hu, X.; Du, D.; Lin, Y. Protein-based nanomaterials and nanosystems for biomedical applications: A review. Mater. Today 2021, 43, 166–184.
- Le Saux, S.; Aubert-Pouëssel, A.; Ouchait, L.; Mohamed, K.E.; Martineau, P.; Guglielmi, L.; Devoisselle, J.-M.; Legrand, P.; Chopineau, J.; Morille, M. Nanotechnologies for Intracellular Protein Delivery: Recent Progress in Inorganic and Organic Nanocarriers. Adv. Ther. 2021, 4, 2100009.
- Zeb, A.; Rana, I.; Choi, H.-I.; Lee, C.-H.; Baek, S.-W.; Lim, C.-W.; Khan, N.; Arif, S.T.; Sahar, N.U.; Alvi, A.M.; et al. Potential and Applications of Nanocarriers for Efficient Delivery of Biopharmaceuticals. Pharmaceutics 2020, 12, 1184.
- Hirai, Y.; Hirose, H.; Imanishi, M.; Asai, T.; Futaki, S. Cytosolic protein delivery using pH-responsive, charge-reversible lipid nanoparticles. Science 2021, 11, 19896.
- Jash, A.; Ubeyitogullari, A.; Rizvi, S.S.H. Liposomes for oral delivery of protein and peptide-based therapeutics: Challenges, formulation strategies, and advances. J. Mater. Chem. B 2021, 9, 4773–4792.
- Mansoor, S.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. Polymer-Based Nanoparticle Strategies for Insulin Delivery. Polymers 2019, 11, 1380.
- Rebekah, A.; Sivaselvam, S.; Viswanathan, C.; Prabhu, D.; Gautam, R.; Ponpandian, N. Magnetic nanoparticle-decorated graphene oxide-chitosan composite as an efficient nanocarrier for protein delivery. Colloids Surf. A Physicochem Eng. Asp. 2021, 610, 125913.
- Abasian, P.; Ghanavati, S.; Rahebi, S.; Nouri Khorasani, S.; Khalili, S. Polymeric nanocarriers in targeted drug delivery systems: A review. Polym. Adv. Technol. 2020, 31, 2939–2954.
- Agrahari, V. Advances and applications of block-copolymer-based nanoformulations. Drug Discov. 2018, 23, 1139–1151.
- Karayianni, M.; Pispas, S. Self-Assembly of Amphiphilic Block Copolymers in Selective Solvents. In Fluorescence Studies of Polymer Containing Systems, 1st ed.; Procházka, K., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 27–63.
- Perin, F.; Motta, A.; Maniglio, D. Amphiphilic copolymers in biomedical applications: Synthesis routes and property control. Mater. Sci. Eng. C 2021, 123, 111952.
- Gao, S.; Holkar, A.; Srivastava, S. Protein-Polyelectrolyte Complexes and Micellar Assemblies. Polymers 2019, 11, 1097.
- Lombardo, D.; Kiselev, M.A.; Caccamo, M.T. Smart Nanoparticles for Drug Delivery Application: Development of Versatile Nanocarrier Platforms in Biotechnology and Nanomedicine. J. Nanomater. 2019, 2019, 3702518.
- Venditti, I. Morphologies and functionalities of polymeric nanocarriers as chemical tools for drug delivery: A review. J. King Saud. Univ. Sci. 2019, 31, 398–411.
- Kopeček, J.; Yang, J. Polymer nanomedicines. Adv. Drug Deliv. Rev. 2020, 156, 40–64.
- Das, S.S.; Bharadwaj, P.; Bilal, M.; Barani, M.; Rahdar, A.; Taboada, P.; Bungau, S.; Kyzas, G.Z. Stimuli-Responsive Polymeric Nanocarriers for Drug Delivery, Imaging, and Theragnosis. Polymers 2020, 12, 1397.
- Zhang, H.; Mi, P. 12—Polymeric Micelles for Tumor Theranostics. In Theranostic Bionanomaterials, 1st ed.; Cui, W., Zhao, X., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 289–302.
- Javan Nikkhah, S.; Thompson, D. Molecular Modelling Guided Modulation of Molecular Shape and Charge for Design of Smart Self-Assembled Polymeric Drug Transporters. Pharmaceutics 2021, 13, 141.
- Lombardo, D.; Kiselev, M.A.; Magazù, S.; Calandra, P. Amphiphiles Self-Assembly: Basic Concepts and Future Perspectives of Supramolecular Approaches. Adv. Condens. Matter Phys. 2015, 2015, 151683.
- Díez-García, I.; Santamaria-Echart, A.; Eceiza, A.; Tercjak, A. Triblock copolymers containing hydrophilic PEO blocks as effective polyols for organic solvent-free waterborne poly(urethane-urea)s. React. Funct Polym. 2018, 131, 1–11.
- El Jundi, A.; Buwalda, S.J.; Bakkour, Y.; Garric, X.; Nottelet, B. Double hydrophilic block copolymers self-assemblies in biomedical applications. Adv. Colloid Interface Sci. 2020, 283, 102213.
- Feng, H.; Lu, X.; Wang, W.; Kang, N.G.; Mays, J.W. Block Copolymers: Synthesis, Self-Assembly, and Applications. Polymers 2017, 9, 494.
- Perrier, S. 50th Anniversary Perspective: RAFT Polymerization—A User Guide. Macromolecules 2017, 50, 7433–7447.
- Fairbanks, B.D.; Gunatillake, P.A.; Meagher, L. Biomedical applications of polymers derived by reversible addition—Fragmentation chain-transfer (RAFT). Adv. Drug Deliv. Rev. 2015, 91, 141–152.
- Truong, N.P.; Jones, G.R.; Bradford, K.G.E.; Konkolewicz, D.; Anastasaki, A. A comparison of RAFT and ATRP methods for controlled radical polymerization. Nat. Rev. Chem. 2021, 5, 859–869.
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block Copolymer Micelles in Nanomedicine Applications. Chem. Rev. 2018, 118, 6844–6892.
- Torres, J.; Dhas, N.; Longhi, M.; García, M.C. Overcoming Biological Barriers with Block Copolymers-Based Self-Assembled Nanocarriers. Recent Advances in Delivery of Anticancer Therapeutics. Front. Pharmacol. 2020, 11, 1840.
- Bodratti, A.M.; Alexandridis, P. Amphiphilic block copolymers in drug delivery: Advances in formulation structure and performance. Expert. Opin. Drug Deliv. 2018, 15, 1085–1104.
- Jiang, Z.; Liu, H.; He, H.; Ribbe, A.E.; Thayumanavan, S. Blended Assemblies of Amphiphilic Random and Block Copolymers for Tunable Encapsulation and Release of Hydrophobic Guest Molecules. Macromolecules 2020, 53, 2713–2723.
- Liu, L.-Y.; Xia, G.; Feng, Z.-J.; Hao, Q.-H.; Tan, H.-G. Self-assembly of polyelectrolyte diblock copolymers at monovalent and multivalent counterions. Soft Matter 2019, 15, 3689–3699.
- Demetzos, C. Application of Nanotechnology in Drug Delivery and Targeting. In Pharmaceutical Nanotechnology: Fundamentals and Practical Applications; Springer: Singapore, 2016; pp. 77–145.
- Jayasuriya, A.C. 8—Production of micro- and nanoscale chitosan particles for biomedical applications. In Chitosan Based Biomaterials Volume 1; Jennings, J.A., Bumgardner, J.D., Eds.; Woodhead Publishing: Sawston, Cambridge, UK, 2017; pp. 185–209.
- Theodorou, A.; Liarou, E.; Haddleton, D.M.; Stavrakaki, I.G.; Skordalidis, P.; Whitfield, R.; Anastasaki, A.; Velonia, K. Protein-polymer bioconjugates via a versatile oxygen tolerant photoinduced controlled radical polymerization approach. Nat. Commun. 2020, 11, 1486.
- Matyjaszewski, K. Advanced Materials by Atom Transfer Radical Polymerization. Adv. Mater. 2018, 30, e1706441.
- Hou, W.; Wei, L.; Liu, L.; Zhao, H. Surface Coassembly of Polymer Brushes and Polymer–Protein Bioconjugates: An Efficient Approach to the Purification of Bioconjugates under Mild Conditions. Biomacromolecules 2018, 19, 4463–4471.
- Wang, Y.; Wu, C. Site-Specific Conjugation of Polymers to Proteins. Biomacromolecules 2018, 19, 1804–1825.
- Abuchowski, A.; van Es, T.; Palczuk, N.C.; Davis, F.F. Alteration of immunological properties of bovine serum albumin by covalent attachment of polyethylene glycol. J. Biol. Chem. 1977, 252, 3578–3581.
- Kariduraganavar, M.Y.; Heggannavar, G.B.; Amado, S.; Mitchell, G.R. Chapter 6—Protein Nanocarriers for Targeted Drug Delivery for Cancer Therapy. In Nanocarriers for Drug Delivery, 1st ed.; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 173–204.
- Rondon, A.; Mahri, S.; Morales-Yanez, F.; Dumoulin, M.; Vanbever, R. Protein Engineering Strategies for Improved Pharmacokinetics. Adv. Funct. Mater. 2021, 31, 2101633.
- Dozier, J.K.; Distefano, M.D. Site-Specific PEGylation of Therapeutic Proteins. Int. J. Mol. Sci. 2015, 16, 25831–25864.
- Harijan, M.; Singh, M. Zwitterionic polymers in drug delivery: A review. J. Mol. Recognit. 2022, 35, e2944.
- Hoang Thi, T.T.; Pilkington, E.H.; Nguyen, D.H.; Lee, J.S.; Park, K.D.; Truong, N.P. The Importance of Poly(ethylene glycol) Alternatives for Overcoming PEG Immunogenicity in Drug Delivery and Bioconjugation. Polymers 2020, 12, 298.
- Ju, Y.; Zhang, Y.; Zhao, H. Fabrication of Polymer–Protein Hybrids. Macromol. Rapid Commun. 2018, 39, 1700737.
- Kurinomaru, T.; Kuwada, K.; Tomita, S.; Kameda, T.; Shiraki, K. Noncovalent PEGylation through Protein–Polyelectrolyte Interaction: Kinetic Experiment and Molecular Dynamics Simulation. J. Phys. Chem. B. 2017, 121, 6785–6791.
- Reichert, C.; Borchard, G. Noncovalent PEGylation, An Innovative Subchapter in the Field of Protein Modification. J. Pharm. Sci. 2016, 105, 386–390.
- Ye, C.; Chi, H. A review of recent progress in drug and protein encapsulation: Approaches, applications and challenges. Mater. Sci. Eng. C 2018, 83, 233–246.
- Li, C.; Wu, G.; Ma, R.; Liu, Y.; Liu, Y.; Lv, J.; An, Y.; Shi, L. Nitrilotriacetic Acid (NTA) and Phenylboronic Acid (PBA) Functionalized Nanogels for Efficient Encapsulation and Controlled Release of Insulin. ACS Biomater. Sci. Eng. 2018, 4, 2007–2017.
- Chen, F.; Stenzel, M.H. Polyion Complex Micelles for Protein Delivery. Aust. J. Chem. 2018, 71, 768–780.
