Anthocyanins are water-soluble, colored compounds of the flavonoid class, abundantly found in the fruits, leaves, roots, and other parts of the plants. The fruit berries are prime sources and exhibit different colors. The anthocyanins utility as traditional medicament for liver protection and cure, and importance as strongest plants-based anti-oxidants have conferred these plants products different biological activities. These activities include anti-inflammation, liver protective, analgesic, and anti-cancers, which have provided the anthocyanins an immense commercial value, and has impelled their chemistry, biological activity, isolation, and quality investigations as prime focus.
- anthocyanins
- traditional medicine
- liver protection
- hepatocellular longevity
- hepatic carcinoma
- anti-oxidant
- anti-inflammation
- NrF2
- TNF-α
1. Introduction
2. Anthocyanins’ Aesthetics, and Plant Kingdom’s Distribution
3. Anthocyanins Roles in the Plants
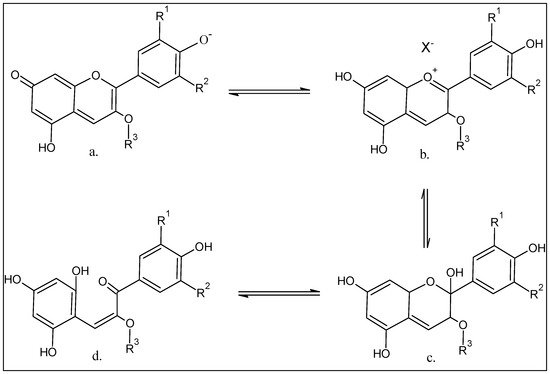
4. Chemistry of the Anthocyanins, Structures, and the Structural Variants
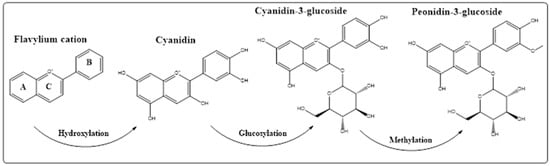
5. Anthocyanins Extraction, Purification, and Structure Determinations
6. Herbal Medicines Traditional Uses, Toxicity, Liver Disorders, and Anthocyanins
|
Plant’s Name |
Folklore Medicinal Uses, Other than Liver Disorders |
Plant Parts Used |
Major Identified Anthocyanins |
Refer |
|---|---|---|---|---|
|
Hibiscus sabdariffa |
Hypertension, pyrexia |
Calyx, Epicalyx |
Cyanidin-3-O-β-glucoside, and delphinidin-3-glucoside |
|
|
Cichorium intybus |
Inflammation |
Leaves |
Cyanidin-3-O-(6″-malonyl-β-glucopyranoside) |
[177] |
|
Garcinia indica |
Male digestion, flatulence, and constipation. |
Fruits |
Cyanidin-3-O-β-glucoside, and cyanidin-3-O-sambubioside |
[178] |
|
Raphanus sativus |
Roots |
Pelargonidin derivatives |
[179] |
|
|
Morus alba (Mulberry), & other species |
Cardiovascular diseases, nephritis, thirsty, constipation |
Fruits |
Cyanidin-3-O-rutinoside, cyanidin-3-O-glucoside |
|
|
Cornus mas (cornelian cherry) |
Diabetes, diarrhea, fevers, rheumatic complains, skin diseases and urinary tract infections |
Fruits |
Cyanidin-3-O-galactoside, pelargonidin-3-O-galactoside, delphinidin-3-O-galactoside, cyanidin-3-O-rutinoside, pelargonidin-3-O-glucoside, pelargonidin-3-O-rutinoside, pegonidin-3-O-glucoside |
[182] |
|
Lannea microcarpa |
Scurvy, rickets and cough. |
Fruits |
Cyanidin-3-O-(2-O-β-D-xylopyranosyl)-β-D-galactopyranoside, and cyanidin-3-O-β-D-galactopyranoside. |
[183] |
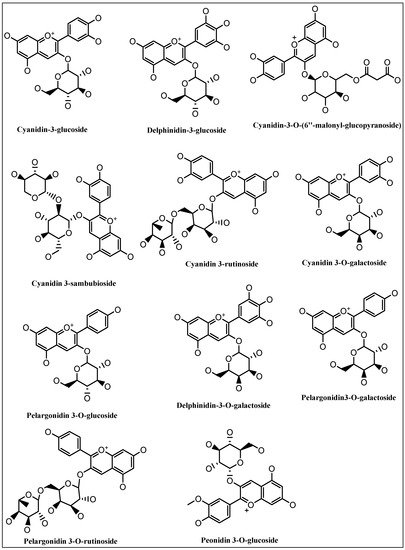
7. Anthocyanins’ Metabolism in Liver
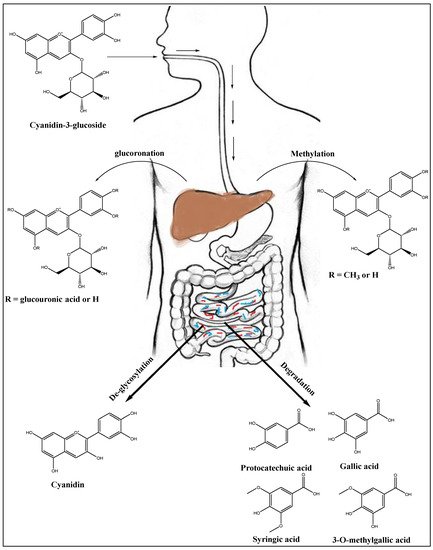
8. Anthocyanins and Liver Disorders
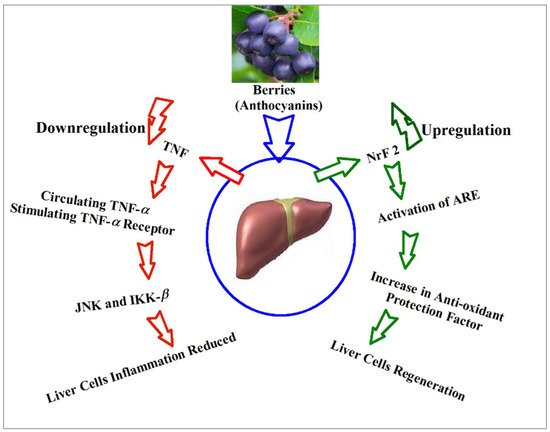
9. Anthocyanins’ Suggestive Roles through Hepatic Biomarkers Regulation, and Biomechanistics Outlook
|
Anthocyanins |
Experimental Protocol |
Mode of Action |
Refer |
|---|---|---|---|
|
Cyanidin-3-O-β-glucoside |
In vivo CCl4-induced liver damage in mice and in vitro H2O2-induced oxidative stress in HepG2 cells apoptosis |
Enhance the antioxidant enzymes activities and upregulating Nrf2-antioxidant pathway. |
[156] |
|
Delphinidin |
In vitro H2O2-induced oxidative stress in HepG2 Cells |
Enhance the expression of Nrf2 and promoted Nrf2 nuclear translocation. Increase expression of antioxidant protein HO-1 (Nrf2-related phase II enzyme heme oxygenase-1). Alleviate the reduction of Nrf2 protein levels and the accumulation of intracellular ROS levels in Nrf2 knockdown HepG2 cells. |
[288] |
|
Mixture of cyanidin-3-O-β-glucoside, delphinidin-3-O-rutinoside, and malvidin-3-O-galactoside |
In vivo CCl4-induced human embryonic-liver (L-02) cells toxicity |
Reduce the percentage of hypo-diploid cells and decrease in caspase-3 protein expression |
[289] |
|
Cyanidin-3-O-β-glucoside, and peonidin-3-O-glucoside |
In vitro human embryo non-malignant liver tissue cell line (L-02). Hepatoprotection |
Exhibited higher cell viability, decreased aminotransferase activity and enhanced cellular antioxidant status. Furthermore, Cy-3-G showed much stronger hepatoprotective activity than Pn-3-G at the same concentration. |
[186] |
|
Plant’s Name |
Used Extracts, and/or Pure Compounds |
In Vivo/In Vitro Models and Bioactivity |
Major Anthocyanins |
Biomarkers, and Mode/Mechanism of Action |
Refer |
|---|---|---|---|---|---|
|
Morus alba, and species |
Mulberry anthocyanins |
CCl4 (carbon tetrachloride), in vivo model. Hepatoprotection |
Cyanidin-3-O-β-glucoside |
Decreased the ALT (alanine transaminase), AST (aspartate transaminase), hyaluronidase, hydroxyproline, and collagen type-III in the injured rats |
[293] |
|
Ipomoea batatas L. |
Anthocyanins rich purple sweet potato extract |
CCl4, in vivo model. Hepatoprotection |
Peonidin-3-caffeoyl-feruloyl sophoroside-5-glucosid, peonidin 3-caffeoyl-p-hydroxy benzoyl sophoroside-5-glucoside, peonidin 3-dicaffeoyl sophoroside-5-glucoside |
Reduced the AST and ALT enzymes and MDA (malondialdehyde) level; Increased the SOD (superoxide dismutase), and GSH (glutathione) levels compared to the injured CCl4 administered group of animals |
[295] |
|
Oryza sativa |
Anthocyanins rich black rice bran extract |
CCl4, in vivo model. Hepatoprotection |
Cyanidin-3-O-β-glucoside, and peonidin-3-O-glucoside |
Reduced aminotransferase activity in serum, enhanced SOD and glutathione peroxidase (GSH-Px) activities, thiobarbituric acid reactive substances (TBARS), and 8-hydroxy-20-deoxyguanosine levels significantly decreased as compared to the CCl4 intoxicated group. Liver histopathology confirmed pathological gains by ARBE administration |
[186] |
|
Ipomoea batatas |
Anthocyanins rich fraction of purple sweet potato extract |
In vivo, ethanol, acetaminophen, and, CCl4. Hepatoprotection, and treatment |
3-O-(6-O-trans-caffeyt-2-O-~-glucopyranosyl/3-glucopyranoside)-5-O-glucosides of cyanidin, and peonidin |
Treatments of mice with anthocyanins fraction in dose dependent manner, and reduced the CYP2E1-dependent aniline hydroxylation, and CYP2E1 protein levels. Antioxidant effects on hepatic GSH level, and GSH S-transferase activity were up-regulated in FeCl2/ascorbate-induced lipid peroxidation in mouse liver homogenates, also showed superoxide radical scavenging activity. |
|
|
Hibiscus sabdariffa L. |
Anthocyanin-rich extract |
In vivo, thioacetamide (TAA)-induced hepatotoxicity. Hepatoprotection |
Cyanidine, delphinidin derivatives, cyanidin-3,5-O-di-glucoside, cyanidin-3-O-sophoroside-5-glucoside |
Reduced the serum levels of ALA, AST, and hepatic malondialdehyde, decreased hepatic inflammatory markers, including TNF-α, interleukin-6, and INF-γ, decreased the immuno-positivity of NF kappa-B, and CYP2E1 in liver tissues |
[185] |
|
In vivo tert-BHP-induced cytotoxicity in rat |
[176] |
||||
|
CCl4 in vivo model. Hepatoprotection |
[296] |
||||
|
Aronia melanocarpa |
Fruit juice |
CCl4, N-nitroso diethyl amine, Paracetamol in vivo model. Hepatoprotection |
Cyanidin-3-O-galactoside, cyanidin-3-O-arabinoside, cyanidin-3-O-xyloside and cyanidin-3-O-β-glucoside |
Reduced necrotic changes in rat liver and inhibited increase of plasma AST and ALT activities, MDA formation induced by CCl4. Increased liver GSH contents. Decreased the activities of enzymatic markers of cytochrome P450, CYP1A1 and 1A2. |
|
|
Justicia spicigera |
Ethyl acetate fraction |
CCl4 in vivo model. Hepatoprotection |
Peonidin 3,5-O-di-glucoside, malvidin 3,5-O-di-glucoside, and petunidin 3,5-O-di-glucoside |
Improvement in liver function indices and oxidative stress markers. Increased SOD and GSH, and decreased MDA. |
[184] |
|
Vaccinium sp. |
Berry pomace extract |
In vitro hepatic cell line HepG2 proliferation. Hepatic cells protection |
Procyanidin dimers |
Protects hepatic cells from oxidative damage. |
[300] |
|
Solanum tuberosum L. |
Purple potato’s anthocyanins rich extract |
In vivo, alcoholic liver disease mouse model. Hepatoprotection |
Petunidin-3-coumaroyl-rutinoside-5-glucoside, peonidin-3-coumaroyl-rutinoside-5-glucoside, petunidin-3-O-glucoside, petunidin-3-rutinoside-5-glucoside, pelphinidin-3-coumaroyl-rutinoside-5-glucoside |
Higher levels of SOD and reduced GSH enzymes, reduction in formation of malondialdehyde, protected against alcohol-induced detrimental levels, maneuvered the activity of cytochrome P450 2E1 (CYP2E1) |
[301] |
|
Ipomoea batatas |
Anthocyanin fraction |
Dimethyl nitrosamine-induced liver injury in rats. Hepatoprotection |
Cyanidin-3-O-β-glucoside chloride, malvidin-3-O-glucoside, pelargonidin-3-O-glucoside chloride, and peonidine-3-O-glucoside chloride |
Induced Nrf2 mediated antioxidant enzymes, and reduced the COX-2, and iNOS expressions, reduced inflammation through NF-KB inhibition |
[302] |
|
Hibiscus sabdariffa |
Water extract, and anthocyanins |
Paracetamol-induced hepatotoxicity in rats. Hepatoprotection |
Anthocyanins |
Increased GSH and SOD levels, decreased ALT and AST |
[303] |
|
Colocasia antiquorum |
Ethanolic extract |
Paracetamol, and CCl4 toxicated rats. Hepatoprotection |
Cyanidin-3-O-β-glucoside, pelargonidin-3-O-glucoside and cyanidin-3-O-rhamnoside |
Decreased ALT, and AST levels |
[304] |
|
Vaccinium myrtillus and Ribes nigrum |
Anthocyanins-rich extracts |
Acetaminophen-induced hepatotoxicity in rats. Hepatoprotection |
Glycosides of cyanidin, peonidin, delphinidin, petunidin, and malvidin |
Normalized activities of glutamate oxaloacetate and glutamate pyruvate transaminase, prevented APAP-induced plasmatic and tissue alterations in biomarkers of oxidative stress |
[305] |
|
Raphanus sativus L. (Red radish) |
Anthocyanins fraction |
CCl4 in vivo model. Hepatoprotection |
Pelargonidin derivatives |
Reversed the alteration of biochemical parameters to normal |
[179] |
|
Raphanus sativus L. var. niger |
Fermented roots |
In vivo model for the methionine, and choline-deficient, diet-induced non-alcoholic fatty liver in mice. Hepatoprotection |
Pelargonidin derivatives |
Decreased lipids in 3T3-L1 adipocytes by downregulating adipogenic transcription factors, sterol regulatory element-binding protein 1c, CCAAT/enhancer-binding protein α, peroxisome proliferator-activated receptor γ, and lipid accumulation-related genes adipocyte protein-2, as well as fatty acid synthase. Decreased ALT, AST, TG levels. Deceased expression of iNO synthase, suppression of the inactivation of macrophages, and Kupffer cells in liver. Inhibition of α-smooth muscle actin, transforming growth factor β-1, and collagen type-I α-1 chain leading to reduced liver fibrosis. |
[306] |
|
Raphanus sativus L. var. niger |
Aqueous extract of roots |
In vitro model in HepG2 cells. Hepatoprotection |
Pelargonidin derivatives |
Induced quinone reductase activity, and expression of multiple phase I, II detoxification enzymes in the HepG2 human hepatoma cell line |
[307] |
|
Malvaviscus arboreus Cav |
Aerial parts extracts |
CCl4 in vivo model. Hepatoprotection |
Cyanidin-3-sambubioside |
EtOAc (ethyl acetate), and CH2Cl2 (Dichloromethane) extracts significantly reduced the liver injury in rats as indicated by the reduced levels of ALT, AST, ALP, TB, and MDA, comparatively the EtOAc fraction enhanced total antioxidant capacity of liver at the maximum. |
[308] |
|
Cornus mas L. |
Anthocyanins rich fraction |
Lipid peroxidation, oxidative stress in the livers of cholesterol-fed rabbits |
Delphinidin 3-O-galactoside, cyanidin-3-O-galactoside, Cyanidin-3-O-robinobioside, pelargonidin-3-O-galactoside, pelargonidin-3-O-robinobioside, cyanidin, and pelargonidin. |
Decreased lipid peroxidation, decreased MDA levels, and reduced oxidative stress, an increase in liver GSH found. |
[309] |
10. Anthocyanins Roles in Hepatocellular Longevity, Hepatic Carcinoma and Liver Cancer
This entry is adapted from the peer-reviewed paper 10.3390/ijms23042149
