Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cell Biology
Calebin A (CA) is one of the active constituents of turmeric and has anti-inflammatory and antioxidant effects. Excessive inflammation and cell apoptosis are the main causes of tendinitis and tendinopathies. However, the role of CA in tendinitis is still unclear and needs to be studied in detail.
- tenocytes
- Calebin A
- tendinitis microenvironment
- T-lymphocytes
1. Introduction
Tendinopathies is a medical term describing various types of tendon injuries, including trauma, rupture, and especially inflammation-related tendinitis, which are occurring more frequently worldwide [1][2] and account for almost one-third of all musculoskeletal consultations [3]. Moreover, tendinopathies are disabling but also very painful conditions that significantly affect the quality of patients’ daily lives [4][5], explaining their strong desire for relief. The standard medical treatment commonly provided to patients comprises oral non-steroidal anti-inflammatory drugs (NSAIDs) [6] and corticosteroid injections [7]. However, their efficacy in reducing pain and inflammation comes with high costs, as these treatments are associated with serious side effects in multiple organs such as the gastrointestinal, cardiovascular, hepatic, renal, cerebral and pulmonary systems [8][9][10]. In addition, a link was shown between NSAIDs application and inhibitory effects on proteoglycan synthesis and tendon cell proliferation [11], as well as the attenuation of collagen during corticosteroid treatment [12][13][14]. These facts emphasize the urgency for a safer and more specific therapy in the treatment of tendinopathies.
The reasons underlying tendinopathies are various and partly go hand in hand, such as the natural process of aging, physical inactivity and a sedentary lifestyle, mechanical stress and overuse, genetic predisposition or a poor diet [15][16], to name just a few. On the other hand, research showed that tendon ruptures and tendinitis can also occur as a consequence of high-performance sports [17][18] or as side effects of certain drug therapies, such as the antibiotic group of quinolones [19][20][21], primarily by destroying the connection between tenocytes and the extracellular matrix (ECM).
Morphologically, tenocytes have a fibroblast-like shape, expressing Scleraxis as a tendon-specific transcription factor [22][23], and they are embedded in the ECM, a complex and highly dynamic network composed of non-cellular components such as collagen (mainly Collagen I), Elastin, Fibronectin and proteoglycans. The ECM, produced by tenocytes themselves, not only determines a tendon’s stability and its mechanical properties, but is also essential for the regulation of cellular functions such as growth, survival, differentiation or the maintenance of homeostasis. These regulatory processes are mediated by cell surface receptors such as the integrin family, which transmit signals from ECM to cells and vice versa [24][25][26][27][28].
In this context, several studies showed that integrins, as heterodimeric transmembrane protein receptors, play a fundamental role in this process. They not only integrate ECM and cytoskeletal structures into a functional unit, but also actively transduce signals into the cell and vice versa, consequently possessing a wide range of functions essential for tissue survival, differentiation, and homeostasis [29][30][31][32]. Recalling the importance of the various functions of integrin and its role of being strongly involved in ECM anchorage signals, thus the survival of cells, it becomes clear that, the disruption of the specific interplay between ECM and cells might cause apoptotic cell death [32][33].
To date, inflammation and pro-inflammatory processes are known to primarily contribute to the pathogenesis of tendinopathy and tendinitis [25], thus being accompanied by catabolic events, ultrastructural changes, and even tenocyte apoptosis. The up-regulation of pro-inflammatory cytokines such as TNF-α or TNF-β, and the activation of catabolic enzymes such as Cyclooxygenase-2 (COX-2) or Matrix-metalloproteinase (MMP) accompanying inflammatory processes, are mostly promoted by the activation of the Nuclear Factor κappa B (NF-κB) pathway, a pro-inflammatory transcription factor engaged in the regulation of various genes [32][34][35][36]. NF-κB usually resides in the cytoplasm of cells, but when activated through its phosphorylation, it translocates into the nucleus. Since NF-κB serves as a central mediator for inflammatory processes and its up-regulation is mostly associated with inflammatory disease, it represents a potential target in treatment strategies [37][38][39].
Recently, the attention towards natural products as promising and effective treatment options has grown, especially in inflammation-triggered disease, since many studies clearly demonstrated their multi-targeting potential and the anti-inflammatory evidence of polyphenols, which is partially and specifically achieved by inhibiting NF-κB [40][41][42][43][44][45]. In fact, in contrast to standard therapy, natural compounds provide a much safer and less expensive treatment with no undesirable side effects [46].
Calebin A (CA) [4-(3-methoxy-4-hydroxyphenyl)-2-oxo-3-enebutanyl 3-(3-methoxy-4-hydroxyphenyl) propenoate], a non-curcuminoid active component obtained from the rhizome of turmeric (Curcuma longa L., Zingiberaceae) [47][48] is described to possess anti-inflammatory, anti-tumor and anti-oxidant properties [47][49][50][51], whereby many of these studies identified a significant suppressing effect of CA on the activation of the pro-inflammatory NF-κB pathway. However, most of the studies were based on the investigation of tumor cells, and only a few were carried out on cells of the connective tissue [49][51][52][53].
2. Calebin A Suppresses TNF-β- Similar to TNF-α-Mediated Adhesiveness of T-Lymphocytes to Tenocytes
It is well known that inflammatory processes in tissues are associated with the recruitment and migration of T-lymphocytes and other leukocytes to the site of inflammation [54]. To investigate whether a similar effect can be detected in tenocytes, researchers performed an adhesion experiment with T-lymphocytes. Tenocytes were grown to subconfluence in monolayer cultures, either by themselves or in co-cultures with T-lymphocytes, treated as outlined in Material and Methods.
Co-culturing tenocytes with T-lymphocytes alone (as control, Co) or with additional treatment with cytokines (such as TNF-α or TNF-β) showed the similar tendency of T-lymphocytes to attach and adhere to tenocytes, which is significantly increased by cytokine stimulation, underlining their role in enhancing T-lymphocyte migration, thus manifesting an inflammatory environment (Figure 1). In contrast, untreated co-cultures, or co-cultures treated with CA or with CA and pro-inflammatory cytokines, showed a remarkable suppression of T-lymphocyte accumulation and adherence to tenocytes with a concentration-dependent effect (Figure 1). Overall, these results indicate the adhesion of T-lymphocytes to tenocytes in a tendinitis environment as one of the main targets of CA for its anti-inflammatory mechanisms in inflammatory environments in tenocyte culture.
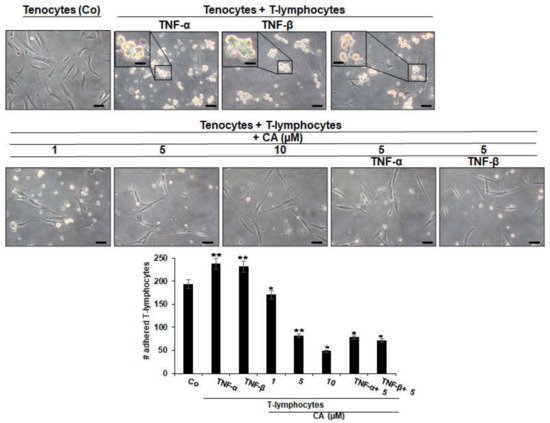
Figure 1. Effect of Calebin A (CA), TNF-α or TNF-β on T-lymphocytes adhesiveness on tenocytes in an inflammatory environment. Serum-starved tenocyte monolayer cultures alone (control, Co), or co-cultured with T-lymphocytes, or co-cultured with T-lymphocytes and TNF-α or TNF-β (10 ng/mL), were either left untreated or treated with various concentrations of CA (1, 5, 10 µM) for 18 h and, subsequently, photographed using a light microscope (Zeiss, Jena, Germany). All assays were performed three times. Data with p < 0.05 (*) and p < 0.01 (**) indicate a significant difference compared to control. Magnification: ×100; scale bar = 30 nm. Insets: magnification: ×400; scale bar = 3 nm.
3. Calebin A Suppresses T-Lymphocyte-Induced NF-κB Phosphorylation/Nuclear Translocation and Down-Regulation of Scleraxis
To analyze whether the above observations of enhanced or suppressed adhesiveness might be associated with the up-regulation of either inflammatory-specific or the down-regulation of tendon-specific proliferating signaling in tenocytes, researchers first performed an immunofluorescence analysis with the master regulator of inflammation NF-κB and the tendon-specific transcription factor, Scleraxis. Tenocytes grown in monolayer on glass plates were either cultured alone (Co) or grown in T-lymphocyte co-cultures and left untreated or treated with CA, as precisely described in Material and Methods. The immunolabeling of the tenocyte control culture showed a moderate expression of NF-κB, while co-culturing with T-lymphocytes resulted in a two-fold increase in NF-κB phosphorylation, and thus the translocation into the nucleus of tenocytes, where it may modulate gene expression. Interestingly, the addition of a moderate concentration of CA to the tenocyte co-culture, clearly showed a down-regulation of NF-κB phosphorylation and a translocation to the nucleus of tenocytes comparable to the initial condition (Co) (Figure 2A). Altogether, these data indicate that CA is able to down-regulate T-lymphocyte-enhanced expression and nuclear translocation of p65-NF-κB to tenocytes in the tendinitis environment, which is at least one of the major mechanisms for its anti-inflammatory effect in tendinitis.
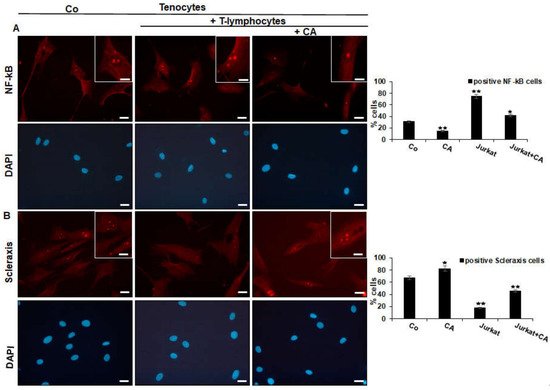
Figure 2. Effect of Calebin A (CA) on NF-κB (A) and on Scleraxis (B) in tenocytes cultured in an inflammatory environment analyzed by immunofluorescence. Serum-starved tenocyte monolayer cultures alone (control, Co) or co-cultured with T-lymphocytes were either left untreated or treated with CA (5 µM) for 5 h. Magnification ×400; scale bar = 45 nm. All experiments were conducted three times and quantification of positively NF-κB- or Scleraxis-labeled nuclei was performed by counting 400–500 cells from 10 different microscopic fields. Values of p < 0.05 (*), p < 0.01 (**) were considered statistically significant in relation to control.
To examine whether the inflammation-protective effect of CA in tenocytes is not only linked with blocking NF-κB activation, but also with triggering tendogenic transcription factor Scleraxis, immunofluorescence labeling against Scleraxis was performed in the same experimental set-up as used for NF-κB (Figure 3B). In the reference control, tenocytes revealed intensive fluorescent labeling of Scleraxis in the nucleus, which was markedly reduced to almost no signaling in the co-culture with T-lymphocytes. In the presence of CA, a strong signaling of Scleraxis was exhibited again in tenocytes co-cultured with T-lymphocytes. Overall, these results suggest that CA may act as an actively multi-targeting component, exerting its effects, at least in part, by suppressing inflammation (NF-κB) and simultaneously activating Scleraxis in an inflammatory environment of tenocytes.
4. Calebin A Suppresses T-Lymphocyte-, TNF-β-, or TNF-α-Promoted ECM Degradation and Signaling Gene Expression, Similar to IKK Inhibitor (BMS-345541) in Tenocytes
To further investigate previous results, suggesting CA to suppress NF-κB translocation into the nucleus and down-regulation of tendon-specific transcription factor Scleraxis, researchers sought to examine whether CA has the capacity to prevent cytokine (TNF-α, TNF-β) or T-lymphocyte induced ECM inhibition by analyzing ECM-specific gene expression (Collagen I, Tenomodulin) in tenocytes.
Samples used for Western blot analysis, consisted of tenocytes cultured by themselves with and without CA treatment (basal controls), or tenocytes grown in co-culture with T-lymphocytes either left untreated or treated with CA (1, 5, 10 µM), treated with cytokines (TNF-α, TNF-β) alone, or concomitantly treated with cytokines and CA (5 µM). Additionally, a sample of tenocytes cultured together with T-lymphocytes and treated with BMS-345541 was analyzed. BMS-345541 is a selective inhibitor of IKK subunits, and thus is able to specifically block NF-κB [55]. Therefore, it was subjected as a control of NF-κB inhibition in the experiment.
Immunoblotting results revealed clearly enhanced expression levels of Collagen I, Tenomodulin, β1-Integrin and Scleraxis in cultures treated with CA, similar to BMS-345541-treated culture (Figure 3A). In contrast, untreated tenocyte cultures in an inflammation-induced environment showed a strong down-regulation of tendon-specific protein expression. Together, these results do not only indicate the strong ability of CA to suppress T-lymphocyte- or cytokine-promoted ECM inhibition in an inflammatory environment, but reveal the inhibition of NF-κB as a major target mechanism of CA to unfold its anti-inflammatory and matrix-stimulating effects in tendinitis-like conditions.
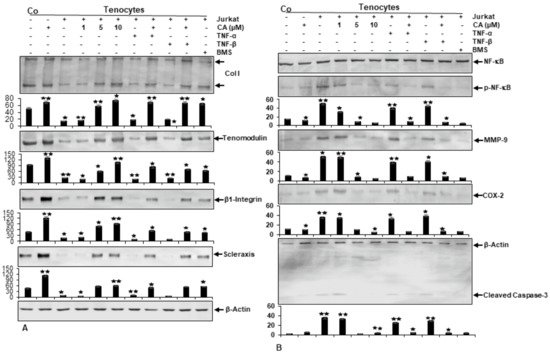
Figure 3. Effect of Calebin A (CA) and IKK-inhibitor BMS-345541 on extracellular matrix and inflammation-associated proteins in tenocyte monolayer cultures. Serum-starved tenocytes in monolayer cultures alone (Co) or co-cultured with T-lymphocytes, or co-cultured with T-lymphocytes and TNF-α or TNF-β (10 ng/mL), were either left untreated or treated with various concentrations of CA (1, 5, 10 µM) or BMS-345541 (5 µM) for 18 h. Western blot analysis was conducted using whole-cell extracts separated by SDS-page, analyzed for (A) Collagen I, Tenomodulin, β1-Integrin, Scleraxis and (B) NF-κB, p-NF-κB, MMP-9, COX-2 and Cleaved Caspase-3, whereby β-Actin served as an internal loading control. Bars demonstrate the mean values of antibodies with the standard deviations of three independent experiments. Values p < 0.05 (*) and p < 0.01 (**) are considered statistically significant in reference to control.
5. Calebin A Suppresses T-Lymphocyte-, TNF-β-, or TNF-α-Promoted NF-κB Activation and NF-κB-Regulated Pro-Inflammatory and Matrix-Degrading Gene Products, Similar to IKK Inhibitor (BMS-345541), in Monolayer Tenocyte Cultures
To gain a deeper insight into previous results suggesting CA could inhibit the activation of NF-κB and its translocation into the nucleus, which is known to stimulate inflammatory processes and up-regulation of catabolic events [32][34][35], they sought to examine the capacity of CA to block inflammation-triggered NF-κB activation in particular, as well as NF-κB promoted gene products taking part in matrix degradation (COX-2, MMP-9) and apoptosis (Cleaved Caspase-3). To investigate if CA suppresses NF-κB activation, tenocyte cultures were analyzed for the phosphorylated form of p65-NF-κB subunits. Immunoblotting revealed higher levels of activated NF-κB expression in the cytokine- and T-lymphocyte-induced inflammatory environment compared to normal control (Figure 3B). Interestingly, in cultures treated with CA, the expression of phosphorylated NF-κB was significantly down-regulated in a concentration-dependent way. In agreement with this observation, NF-κB promoted gene products (MMP-9, COX-2, Cleaved Caspase-3) and showed a decreasing expression level with an increasing CA treatment concentration in inflammation-induced tenocyte environment (Figure 3B). These findings underline previous results and suggest that CA acts as an inhibitor of NF-κB activation by exerting its anti-inflammatory effects, further suppressing the NF-κB pathway-dependent, inflammation-associated (COX-2), matrix-degrading (MMP-9) and apoptosis-related (Cleaved Caspase-3) gene products in an inflammatory tenocyte environment.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23031695
References
- Stephenson, A.L.; Wu, W.; Cortes, D.; Rochon, P.A. Tendon Injury and Fluoroquinolone Use: A Systematic Review. Drug Saf. 2013, 36, 709–721.
- Sendzik, J.; Shakibaei, M.; Schäfer-Korting, M.; Lode, H.; Stahlmann, R. Synergistic effects of dexamethasone and quinolones on human-derived tendon cells. Int. J. Antimicrob. Agents 2010, 35, 366–374.
- Andarawis-Puri, N.; Flatow, E.L.; Soslowsky, L.J. Tendon basic science: Development, repair, regeneration, and healing. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2015, 33, 780–784.
- Childress, M.A.; Beutler, A. Management of chronic tendon injuries. Am. Fam. Physician 2013, 87, 486–490.
- D’Addona, A.; Maffulli, N.; Formisano, S.; Rosa, D. Inflammation in tendinopathy. Surg. J. R. Coll. Surg. Edinb. Irel. 2017, 15, 297–302.
- Bernard-Beaubois, K.; Hecquet, C.; Houcine, O.; Hayem, G.; Adolphe, M. Culture and characterization of juvenile rabbit tenocytes. Cell Biol. Toxicol. 1997, 13, 103–113.
- Chianca, V.; Albano, D.; Messina, C.; Midiri, F.; Mauri, G.; Aliprandi, A.; Catapano, M.; Pescatori, L.C.; Monaco, C.G.; Gitto, S.; et al. Rotator cuff calcific tendinopathy: From diagnosis to treatment. Acta Bio-Med. Atenei Parm. 2018, 89, 186–196.
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147.
- Süleyman, H.; Demircan, B.; Karagöz, Y. Anti-inflammatory and side effects of cyclooxygenase inhibitors. Pharmacol. Rep. PR 2007, 59, 247–258.
- Bacchi, S.; Palumbo, P.; Sponta, A.; Coppolino, M.F. Clinical pharmacology of non-steroidal anti-inflammatory drugs: A review. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2012, 11, 52–64.
- Riley, G.P.; Cox, M.; Harrall, R.L.; Clements, S.; Hazleman, B.L. Inhibition of tendon cell proliferation and matrix glycosaminoglycan synthesis by non-steroidal anti-inflammatory drugs in vitro. J. Hand Surg. 2001, 26, 224–228.
- Mikolyzk, D.K.; Wei, A.S.; Tonino, P.; Marra, G.; Williams, D.A.; Himes, R.D.; Wezeman, F.H.; Callaci, J.J. Effect of corticosteroids on the biomechanical strength of rat rotator cuff tendon. J. Bone Jt. Surgery. Am. Vol. 2009, 91, 1172–1180.
- Tillander, B.; Franzén, L.E.; Karlsson, M.H.; Norlin, R. Effect of steroid injections on the rotator cuff: An experimental study in rats. J. Shoulder Elb. Surg. 1999, 8, 271–274.
- Akpinar, S.; Hersekli, M.A.; Demirors, H.; Tandogan, R.N.; Kayaselcuk, F. Effects of methylprednisolone and betamethasone injections on the rotator cuff: An experimental study in rats. Adv. Ther. 2002, 19, 194–201.
- Rolf, C.; Movin, T. Etiology, histopathology, and outcome of surgery in achillodynia. Foot Ankle Int. 1997, 18, 565–569.
- Dakin, S.G.; Newton, J.; Martinez, F.O.; Hedley, R.; Gwilym, S.; Jones, N.; Reid, H.A.B.; Wood, S.; Wells, G.; Appleton, L.; et al. Chronic inflammation is a feature of Achilles tendinopathy and rupture. Br. J. Sports Med. 2018, 52, 359–367.
- Florit, D.; Pedret, C.; Casals, M.; Malliaras, P.; Sugimoto, D.; Rodas, G. Incidence of Tendinopathy in Team Sports in a Multidisciplinary Sports Club Over 8 Seasons. J. Sports Sci. Med. 2019, 18, 780–788.
- Minghelli, B.; Cadete, J. Epidemiology of musculoskeletal injuries in tennis players: Risk factors. J. Sports Med. Phys. Fit. 2019, 59, 2045–2052.
- Hayes, D.W., Jr.; Gilbertson, E.K.; Mandracchia, V.J.; Dolphin, T.F. Tendon pathology in the foot. The use of corticosteroid injection therapy. Clin. Podiatr. Med. Surg. 2000, 17, 723–735.
- Sendzik, J.; Lode, H.; Stahlmann, R. Quinolone-induced arthropathy: An update focusing on new mechanistic and clinical data. Int. J. Antimicrob. Agents 2009, 33, 194–200.
- Van der Linden, P.D.; Sturkenboom, M.C.; Herings, R.M.; Leufkens, H.G.; Stricker, B.H. Fluoroquinolones and risk of Achilles tendon disorders: Case-control study. BMJ Clin. Res. Ed. 2002, 324, 1306–1307.
- Li, Y.; Ramcharan, M.; Zhou, Z.; Leong, D.J.; Akinbiyi, T.; Majeska, R.J.; Sun, H.B. The Role of Scleraxis in Fate Determination of Mesenchymal Stem Cells for Tenocyte Differentiation. Sci. Rep. 2015, 5, 13149.
- Best, K.T.; Loiselle, A.E. Scleraxis lineage cells contribute to organized bridging tissue during tendon healing and identify a subpopulation of resident tendon cells. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 8578–8587.
- Kannus, P. Structure of the tendon connective tissue. Scand. J. Med. Sci. Sports 2000, 10, 312–320.
- Buhrmann, C.; Mobasheri, A.; Busch, F.; Aldinger, C.; Stahlmann, R.; Montaseri, A.; Shakibaei, M. Curcumin modulates nuclear factor kappaB (NF-kappaB)-mediated inflammation in human tenocytes in vitro: Role of the phosphatidylinositol 3-kinase/Akt pathway. J. Biol. Chem. 2011, 286, 28556–28566.
- Wang, J.H.; Guo, Q.; Li, B. Tendon biomechanics and mechanobiology—A minireview of basic concepts and recent advancements. J. Hand Ther. Off. J. Am. Soc. Hand Ther. 2012, 25, 133–140; quiz 141.
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27.
- Giancotti, F.G.; Ruoslahti, E. Integrin signaling. Science 1999, 285, 1028–1032.
- Loeser, R.F. Integrins and chondrocyte-matrix interactions in articular cartilage. Matrix Biol. J. Int. Soc. Matrix Biol. 2014, 39, 11–16.
- Moser, M.; Legate, K.R.; Zent, R.; Fässler, R. The tail of integrins, talin, and kindlins. Science 2009, 324, 895–899.
- Schwartz, M.A.; Ginsberg, M.H. Networks and crosstalk: Integrin signalling spreads. Nat. Cell Biol. 2002, 4, E65–E68.
- Shakibaei, M.; Csaki, C.; Mobasheri, A. Diverse roles of integrin receptors in articular cartilage. Adv. Anat. Embryol. Cell Biol. 2008, 197, 1–60.
- Mobasheri, A.; Carter, S.D.; Martín-Vasallo, P.; Shakibaei, M. Integrins and stretch activated ion channels; putative components of functional cell surface mechanoreceptors in articular chondrocytes. Cell Biol. Int. 2002, 26, 1–18.
- Busch, F.; Mobasheri, A.; Shayan, P.; Lueders, C.; Stahlmann, R.; Shakibaei, M. Resveratrol modulates interleukin-1β-induced phosphatidylinositol 3-kinase and nuclear factor κB signaling pathways in human tenocytes. J. Biol. Chem. 2012, 287, 38050–38063.
- Hoesel, B.; Schmid, J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 86.
- Mitchell, J.P.; Carmody, R.J. NF-κB and the Transcriptional Control of Inflammation. Int. Rev. Cell Mol. Biol. 2018, 335, 41–84.
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023.
- Ahn, K.S.; Aggarwal, B.B. Transcription factor NF-kappaB: A sensor for smoke and stress signals. Ann. N. Y. Acad. Sci. 2005, 1056, 218–233.
- Aggarwal, B.B.; Takada, Y. Pro-apototic and anti-apoptotic effects of tumor necrosis factor in tumor cells. Role of nuclear transcription factor NF-kappaB. Cancer Treat. Res. 2005, 126, 103–127.
- Das, S.; Das, D.K. Anti-inflammatory responses of resveratrol. Inflamm. Allergy Drug Targets 2007, 6, 168–173.
- Shakibaei, M.; Csaki, C.; Nebrich, S.; Mobasheri, A. Resveratrol suppresses interleukin-1beta-induced inflammatory signaling and apoptosis in human articular chondrocytes: Potential for use as a novel nutraceutical for the treatment of osteoarthritis. Biochem. Pharmacol. 2008, 76, 1426–1439.
- Shakibaei, M.; Buhrmann, C.; Mobasheri, A. Resveratrol-mediated SIRT-1 interactions with p300 modulate receptor activator of NF-kappaB ligand (RANKL) activation of NF-kappaB signaling and inhibit osteoclastogenesis in bone-derived cells. J. Biol. Chem. 2011, 286, 11492–11505.
- Csaki, C.; Mobasheri, A.; Shakibaei, M. Synergistic chondroprotective effects of curcumin and resveratrol in human articular chondrocytes: Inhibition of IL-1beta-induced NF-kappaB-mediated inflammation and apoptosis. Arthritis Res. Ther. 2009, 11, R165.
- He, Y.; Yue, Y.; Zheng, X.; Zhang, K.; Chen, S.; Du, Z. Curcumin, inflammation, and chronic diseases: How are they linked? Molecules 2015, 20, 9183–9213.
- Güleç, A.; Türk, Y.; Aydin, B.K.; Erkoçak, Ö.F.; Safalı, S.; Ugurluoglu, C. Effect of curcumin on tendon healing: An experimental study in a rat model of Achilles tendon injury. Int. Orthop. 2018, 42, 1905–1910.
- Huang, X.M.; Yang, Z.J.; Xie, Q.; Zhang, Z.K.; Zhang, H.; Ma, J.Y. Natural products for treating colorectal cancer: A mechanistic review. Biomed. Pharmacother. 2019, 117, 109142.
- Nair, A.; Amalraj, A.; Jacob, J.; Kunnumakkara, A.B.; Gopi, S. Non-Curcuminoids from Turmeric and Their Potential in Cancer Therapy and Anticancer Drug Delivery Formulations. Biomolecules 2019, 9, 13.
- Park, S.Y.; Kim, D.S. Discovery of natural products from Curcuma longa that protect cells from beta-amyloid insult: A drug discovery effort against Alzheimer’s disease. J. Nat. Prod. 2002, 65, 1227–1231.
- Tyagi, A.K.; Prasad, S.; Majeed, M.; Aggarwal, B.B. Calebin A downregulates osteoclastogenesis through suppression of RANKL signalling. Arch. Biochem. Biophys. 2016, 593, 80–89.
- Aggarwal, B.B.; Yuan, W.; Li, S.; Gupta, S.C. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: Identification of novel components of turmeric. Mol. Nutr. Food Res. 2013, 57, 1529–1542.
- Buhrmann, C.; Popper, B.; Kunnumakkara, A.B.; Aggarwal, B.B.; Shakibaei, M. Evidence That Calebin A, a Component of Curcuma Longa Suppresses NF-B Mediated Proliferation, Invasion and Metastasis of Human Colorectal Cancer Induced by TNF-β (Lymphotoxin). Nutrients 2019, 11, 2904.
- Tyagi, A.K.; Prasad, S.; Majeed, M.; Aggarwal, B.B. Calebin A, a novel component of turmeric, suppresses NF-κB regulated cell survival and inflammatory gene products leading to inhibition of cell growth and chemosensitization. Phytomedicine Int. J. Phytother. Phytopharm. 2017, 34, 171–181.
- Buhrmann, C.; Shayan, P.; Banik, K.; Kunnumakkara, A.B.; Kubatka, P.; Koklesova, L.; Shakibaei, M. Targeting NF-κB Signaling by Calebin A, a Compound of Turmeric, in Multicellular Tumor Microenvironment: Potential Role of Apoptosis Induction in CRC Cells. Biomedicines 2020, 8, 236.
- Chimen, M.; Apta, B.H.; McGettrick, H.M. Introduction: T Cell Trafficking in Inflammation and Immunity. Methods Mol. Biol. 2017, 1591, 73–84.
- Burke, J.R.; Pattoli, M.A.; Gregor, K.R.; Brassil, P.J.; MacMaster, J.F.; McIntyre, K.W.; Yang, X.; Iotzova, V.S.; Clarke, W.; Strnad, J.; et al. BMS-345541 is a highly selective inhibitor of I kappa B kinase that binds at an allosteric site of the enzyme and blocks NF-kappa B-dependent transcription in mice. J. Biol. Chem. 2003, 278, 1450–1456.
This entry is offline, you can click here to edit this entry!
