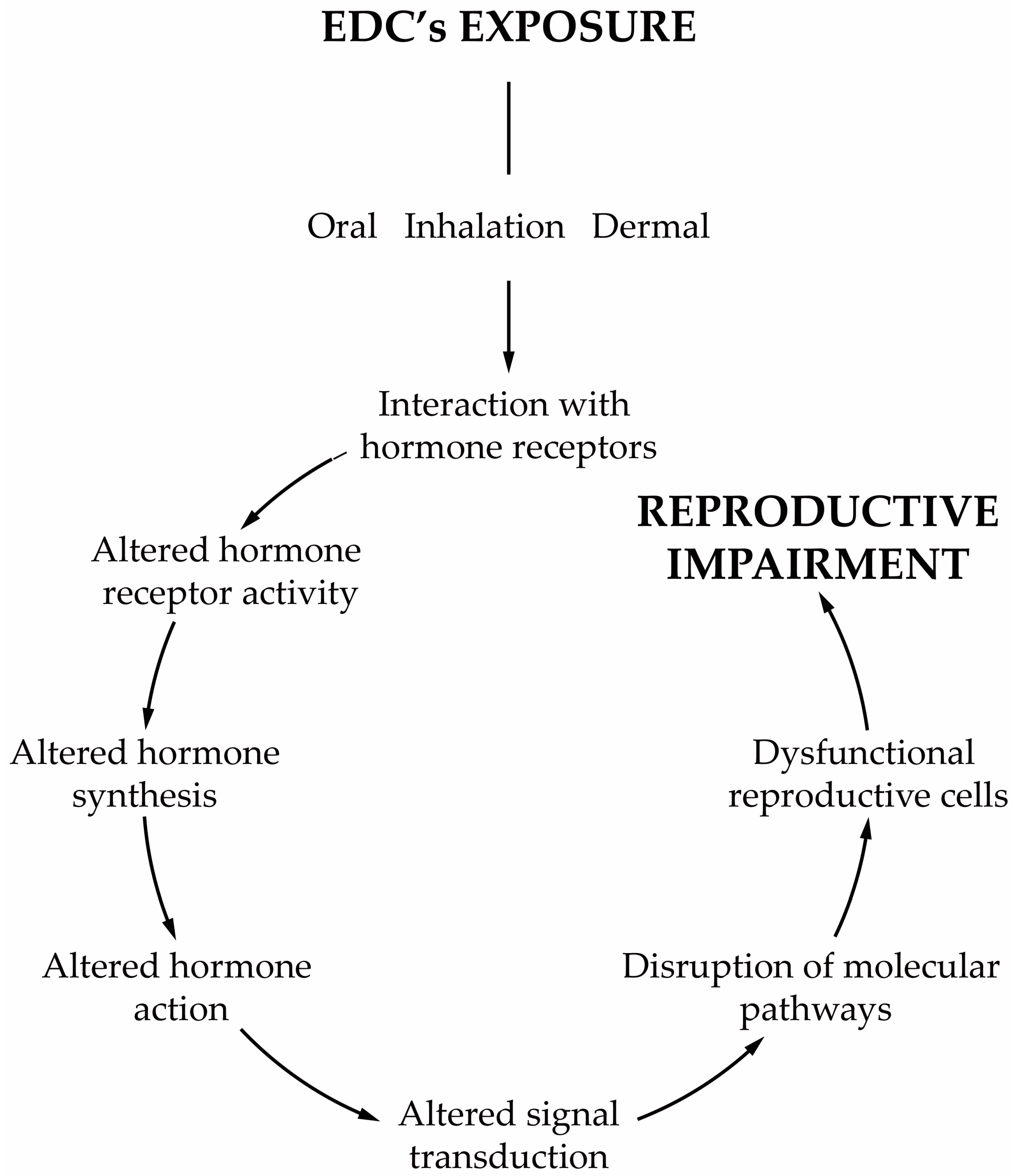
Any compound or mixture of compounds, which interferes with any aspect of the endocrine system is classified as an endocrine-disrupting chemical (EDC). There is increasing evidence that male infertility is associated with the widespread exposure to EDCs, and in particular to obesogens. These compounds interfere with hormones involved in the regulation of metabolism and are associated with weight gain, being also able to change the functioning of the male reproductive axis and, consequently, the testicular physiology and metabolism that are pivotal for spermatogenesis. The disruption of these tightly regulated metabolic pathways leads to adverse reproductive outcomes. The permanent exposure to obesogens has raised serious health concerns. Evidence suggests that obesogens are one of the leading causes of the marked decline of male fertility and key players in shaping the future health outcomes not only for those who are directly exposed but also for upcoming generations. In addition to the changes that lead to inefficient functioning of the male gametes, obesogens induce alterations that are “imprinted” on the genes of the male gametes, establishing a link between generations and contributing to the transmission of defects.
- environmental contaminants
- obesogens
- infertility
- reproductive axis
- spermatogenesis
- Sertoli cells
- germ cells
1. Introduction
2. Obesogens as a Threat to Male Fertility
3. Reproductive Axis at the Interface with Environmental Obesogens
4. Leydig Cells Are a Sensitive Target of Obesogens
5. Mechanisms Mediating Obesogen-Related Sertoli Cell Dysfunction: A Metabolic Standpoint
6. How Can Germ Cells Be Affected by Obesogens?
This entry is adapted from the peer-reviewed paper 10.3390/jox11040012
References
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, E1–E150.
- Grun, F.; Watanabe, H.; Zamanian, Z.; Maeda, L.; Arima, K.; Cubacha, R.; Gardiner, D.M.; Kanno, J.; Iguchi, T.; Blumberg, B. Endocrine-disrupting organotin compounds are potent inducers of adipogenesis in vertebrates. Mol. Endocrinol. 2006, 20, 2141–2155.
- Grün, F.; Blumberg, B. Environmental obesogens: Organotins and endocrine disruption via nuclear receptor signaling. Endocrinology 2006, 147, s50–s55.
- Trasande, L.; Zoeller, R.T.; Hass, U.; Kortenkamp, A.; Grandjean, P.; Myers, J.P.; DiGangi, J.; Bellanger, M.; Hauser, R.; Legler, J.; et al. Estimating burden and disease costs of exposure to endocrine-disrupting chemicals in the European union. J. Clin. Endocrinol. Metab. 2015, 100, 1245–1255.
- Trasande, L. Sicker, Fatter, Poorer: The Urgent Threat of Hormone-Disrupting Chemicals on Our Health and Future... and What We Can Do about It; Houghton Mifflin: Boston, MA, USA, 2019.
- Bruce, B.; Kristin, L. The Obesogen Effect, 1st ed.; Grand Central Life & Style: New York, NY, USA, 2018; p. 307.
- Darbre, P.D. Endocrine Disruptors and Obesity. Curr. Obes. Rep. 2017, 6, 18–27.
- Veiga-Lopez, A.; Pu, Y.; Gingrich, J.; Padmanabhan, V. Obesogenic Endocrine Disrupting Chemicals: Identifying Knowledge Gaps. Trends Endocrinol. Metab. 2018, 29, 607–625.
- Heindel, J.J.; Blumberg, B. Environmental Obesogens: Mechanisms and Controversies. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 89–106.
- Chamorro-Garcia, R.; Blumberg, B. Current Research Approaches and Challenges in the Obesogen Field. Front. Endocrinol. 2019, 10, 167.
- Heindel, J.J. History of the Obesogen Field: Looking Back to Look Forward. Front. Endocrinol. 2019, 10, 14.
- Legler, J.; Fletcher, T.; Govarts, E.; Porta, M.; Blumberg, B.; Heindel, J.J.; Trasande, L. Obesity, diabetes, and associated costs of exposure to endocrine-disrupting chemicals in the European Union. J. Clin. Endocrinol. Metab. 2015, 100, 1278–1288.
- Alonso-Magdalena, P.; Quesada, I.; Nadal, A. Endocrine disruptors in the etiology of type 2 diabetes mellitus. Nat. Rev. Endocrinol 2011, 7, 346–353.
- Kuo, C.C.; Moon, K.; Thayer, K.A.; Navas-Acien, A. Environmental chemicals and type 2 diabetes: An updated systematic review of the epidemiologic evidence. Curr. Diab. Rep. 2013, 13, 831–849.
- Braun, J.M. Early-life exposure to EDCs: Role in childhood obesity and neurodevelopment. Nat. Rev. Endocrinol. 2017, 13, 161–173.
- Fallara, G.; Cazzaniga, W.; Boeri, L.; Capogrosso, P.; Candela, L.; Pozzi, E.; Belladelli, F.; Schifano, N.; Ventimiglia, E.; Abbate, C. Male factor infertility trends throughout the last 10 years: Report from a tertiary-referral academic andrology centre. Andrology 2021, 9, 610–617.
- Rodprasert, W.; Main, K.M.; Toppari, J.; Virtanen, H.E. Associations between male reproductive health and exposure to endocrine-disrupting chemicals. Curr. Opin. Endocr. Metab. Res. 2019, 7, 49–61.
- Wong, E.W.; Cheng, C.Y. Impacts of environmental toxicants on male reproductive dysfunction. Trends Pharmacol. Sci. 2011, 32, 290–299.
- Mathur, P.P.; D’Cruz, S.C. The effect of environmental contaminants on testicular function. Asian J. Androl. 2011, 13, 585–591.
- De Coster, S.; van Larebeke, N. Endocrine-disrupting chemicals: Associated disorders and mechanisms of action. J. Environ. Public Health 2012, 2012, 713696.
- Kelce, W.R.; Wilson, E.M. Environmental antiandrogens: Developmental effects, molecular mechanisms, and clinical implications. J. Mol. Med. 1997, 75, 198–207.
- Sohoni, P.; Sumpter, J. Several environmental oestrogens are also anti-androgens. J. Endocrinol. 1998, 158, 327–339.
- Phillips, K.P.; Foster, W.G. Key developments in endocrine disrupter research and human health. J. Toxicol. Environ. Health B 2008, 11, 322–344.
- Whitehead, S.A.; Rice, S. Endocrine-disrupting chemicals as modulators of sex steroid synthesis. Best Pract. Res. Clin. Endocrinol. Metab. 2006, 20, 45–61.
- Ratcliffe, D.A. Decrease in eggshell weight in certain birds of prey. Nature 1967, 215, 208–210.
- Trasande, L.; Zoeller, R.T.; Hass, U.; Kortenkamp, A.; Grandjean, P.; Myers, J.P.; DiGangi, J.; Hunt, P.M.; Rudel, R.; Sathyanarayana, S.; et al. Burden of disease and costs of exposure to endocrine disrupting chemicals in the European Union: An updated analysis. Andrology 2016, 4, 565–572.
- Attina, T.M.; Hauser, R.; Sathyanarayana, S.; Hunt, P.A.; Bourguignon, J.P.; Myers, J.P.; DiGangi, J.; Zoeller, R.T.; Trasande, L. Exposure to endocrine-disrupting chemicals in the USA: A population-based disease burden and cost analysis. Lancet Diabetes Endocrinol. 2016, 4, 996–1003.
- Reame, V.; Pytlowanciv, E.Z.; Ribeiro, D.L.; Pissolato, T.F.; Taboga, S.R.; Góes, R.M.; Pinto-Fochi, M.E. Obesogenic environment by excess of dietary fats in different phases of development reduces spermatic efficiency of Wistar rats at adulthood: Correlations with metabolic status. Biol. Reprod. 2014, 91, 151–161.
- Wan, H.-T.; Mruk, D.D.; Wong, C.K.; Cheng, C.Y. The apical ES–BTB–BM functional axis is an emerging target for toxicant-induced infertility. Trends Mol. Med. 2013, 19, 396–405.
- Cheng, C.Y.; Mruk, D.D. A local autocrine axis in the testes that regulates spermatogenesis. Nat. Rev. Endocrinol. 2010, 6, 380–395.
- Walker, W.H.; Cheng, J. FSH and testosterone signaling in Sertoli cells. Reproduction 2005, 130, 15–28.
- Rommerts, F.F.; de Jong, F.H.; Brinkmann, A.O.; van der Molen, H.J. Development and cellular localization of rat testicular aromatase activity. J. Reprod. Fertil. 1982, 65, 281–288.
- Gore, A.C. Developmental programming and endocrine disruptor effects on reproductive neuroendocrine systems. Front. Neuroendocrinol. 2008, 29, 358–374.
- Gore, A.C. Organochlorine pesticides directly regulate gonadotropin-releasing hormone gene expression and biosynthesis in the GT1-7 hypothalamic cell line. Mol. Cell. Endocrinol. 2002, 192, 157–170.
- Stoker, T.E.; Parks, L.G.; Gray, L.E.; Cooper, R.L. Endocrine-disrupting chemicals: Prepubertal exposures and effects on sexual maturation and thyroid function in the male rat. A focus on the EDSTAC Recommendations. Crit. Rev. Toxicol. 2000, 30, 197–252.
- Harada, Y.; Tanaka, N.; Ichikawa, M.; Kamijo, Y.; Sugiyama, E.; Gonzalez, F.J.; Aoyama, T. PPARα-dependent cholesterol/testosterone disruption in Leydig cells mediates 2, 4-dichlorophenoxyacetic acid-induced testicular toxicity in mice. Arch. Toxicol. 2016, 12, 1–11.
- Barlow, N.J.; Phillips, S.L.; Wallace, D.G.; Sar, M.; Gaido, K.W.; Foster, P.M. Quantitative changes in gene expression in fetal rat testes following exposure to di (n-butyl) phthalate. Toxicol. Sci. 2003, 73, 431–441.
- Hu, J.; Zhang, Z.; Shen, W.-J.; Azhar, S. Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones. Nutr. Metab. 2010, 7, 1–25.
- Desdoits-Lethimonier, C.; Albert, O.; Le Bizec, B.; Perdu, E.; Zalko, D.; Courant, F.; Lesne, L.; Guille, F.; Dejucq-Rainsford, N.; Jegou, B. Human testis steroidogenesis is inhibited by phthalates. Hum. Reprod. 2012, 27, 1451–1459.
- Nakamura, D.; Yanagiba, Y.; Duan, Z.; Ito, Y.; Okamura, A.; Asaeda, N.; Tagawa, Y.; Li, C.; Taya, K.; Zhang, S.-Y. Bisphenol A may cause testosterone reduction by adversely affecting both testis and pituitary systems similar to estradiol. Toxicol. Lett. 2010, 194, 16–25.
- Alves, M.G.; Rato, L.; Carvalho, R.A.; Moreira, P.I.; Socorro, S.; Oliveira, P.F. Hormonal control of Sertoli cell metabolism regulates spermatogenesis. Cell. Mol. Life Sci. 2013, 70, 777–793.
- Rato, L.; Meneses, M.J.; Silva, B.M.; Sousa, M.; Alves, M.G.; Oliveira, P.F. New insights on hormones and factors that modulate Sertoli cell metabolism. Histol. Histopathol. 2016, 31, 499–513.
- Cardoso, A.M.; Alves, M.G.; Mathur, P.P.; Oliveira, P.F.; Cavaco, J.E.; Rato, L. Obesogens and male fertility. Obes. Rev. 2017, 18, 109–125.
- Foster, P.; Foster, J.R.; Cook, M.W.; Thomas, L.V.; Gangolli, S.D. Changes in ultrastructure and cytochemical localization of zinc in rat testis following the administration of Di-n-pentyl phthalate. Toxicol. Appl. Pharmacol. 1982, 63, 120–132.
- Li, M.W.; Mruk, D.D.; Lee, W.M.; Cheng, C.Y. Disruption of the blood-testis barrier integrity by bisphenol A in vitro: Is this a suitable model for studying blood-testis barrier dynamics? Int. J. Biochem. Cell Biol. 2009, 41, 2302–2314.
- Siu, E.R.; Mruk, D.D.; Porto, C.S.; Cheng, C.Y. Cadmium-induced testicular injury. Toxicol. Appl. Pharmacol. 2009, 238, 240–249.
- Gao, Y.; Mruk, D.D.; Cheng, C.Y. Sertoli cells are the target of environmental toxicants in the testis–a mechanistic and therapeutic insight. Expert Opin. Ther. Targets 2015, 19, 1073–1090.
- Cheng, C.Y. Toxicants target cell junctions in the testis: Insights from the indazole-carboxylic acid model. Spermatogenesis 2014, 4, e981485.
- Su, L.; Mruk, D.D.; Cheng, C.Y. Regulation of drug transporters in the testis by environmental toxicant cadmium, steroids and cytokines. Spermatogenesis 2012, 2, 285–293.
- Cheng, C.Y.; Wong, E.W.; Lie, P.P.; Li, M.W.; Su, L.; Siu, E.R.; Yan, H.H.; Mannu, J.; Mathur, P.P.; Bonanomi, M. Environmental toxicants and male reproductive function. Spermatogenesis 2011, 1, 2–13.
- Xiao, X.; Mruk, D.D.; Tang, E.I.; Wong, C.K.; Lee, W.M.; John, C.M.; Turek, P.J.; Silvestrini, B.; Cheng, C.Y. Environmental toxicants perturb human Sertoli cell adhesive function via changes in F-actin organization mediated by actin regulatory proteins. Hum. Reprod. 2014, 29, 1279–1291.
- Mruk, D.D.; Cheng, C.Y. Environmental contaminants: Is male reproductive health at risk? Spermatogenesis 2011, 1, 283–290.
- Skakkebaek, N.; Rajpert-De Meyts, E.; Main, K. Testicular dysgenesis syndrome: An increasingly common developmental disorder with environmental aspects: Opinion. Hum. Reprod. 2001, 16, 972–978.
- Mitra, S.; Srivastava, A.; Khandelwal, S. Tributyltin chloride induced testicular toxicity by JNK and p38 activation, redox imbalance and cell death in sertoli-germ cell co-culture. Toxicology 2013, 314, 39–50.
- Weston, C.R.; Davis, R.J. The JNK signal transduction pathway. Curr. Opin. Cell Biol. 2007, 19, 142–149.
- Urushibara, N.; Mitsuhashi, S.; Sasaki, T.; Kasai, H.; Yoshimizu, M.; Fujita, H.; Oda, A. JNK and p38 MAPK are independently involved in tributyltin-mediated cell death in rainbow trout (Oncorhynchus mykiss) RTG-2 cells. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2009, 149, 468–475.
