The gut microbiota has been designated as a hidden metabolic ‘organ’ because of its enormous impact on host metabolism, physiology, nutrition, and immune function. The connection between the intestinal microbiota and their respective host animals is dynamic and, in general, mutually beneficial. This complicated interaction is seen as a determinant of health and disease; thus, intestinal dysbiosis is linked with several metabolic diseases. Therefore, tractable strategies targeting the regulation of intestinal microbiota can control several diseases that are closely related to inflammatory and metabolic disorders. As a result, animal health and performance are improved. One of these strategies is related to dietary supplementation with prebiotics, probiotics, and phytogenic substances. These supplements exert their effects indirectly through manipulation of gut microbiota quality and improvement in intestinal epithelial barrier.
- gut microbiota
- dysbiosis
- tight junctions
- synbiotics
- poultry
- feed additives
1. Introduction
2. Intestinal Microbiota in Poultry
3. Intestinal Barrier and Tight Junctions
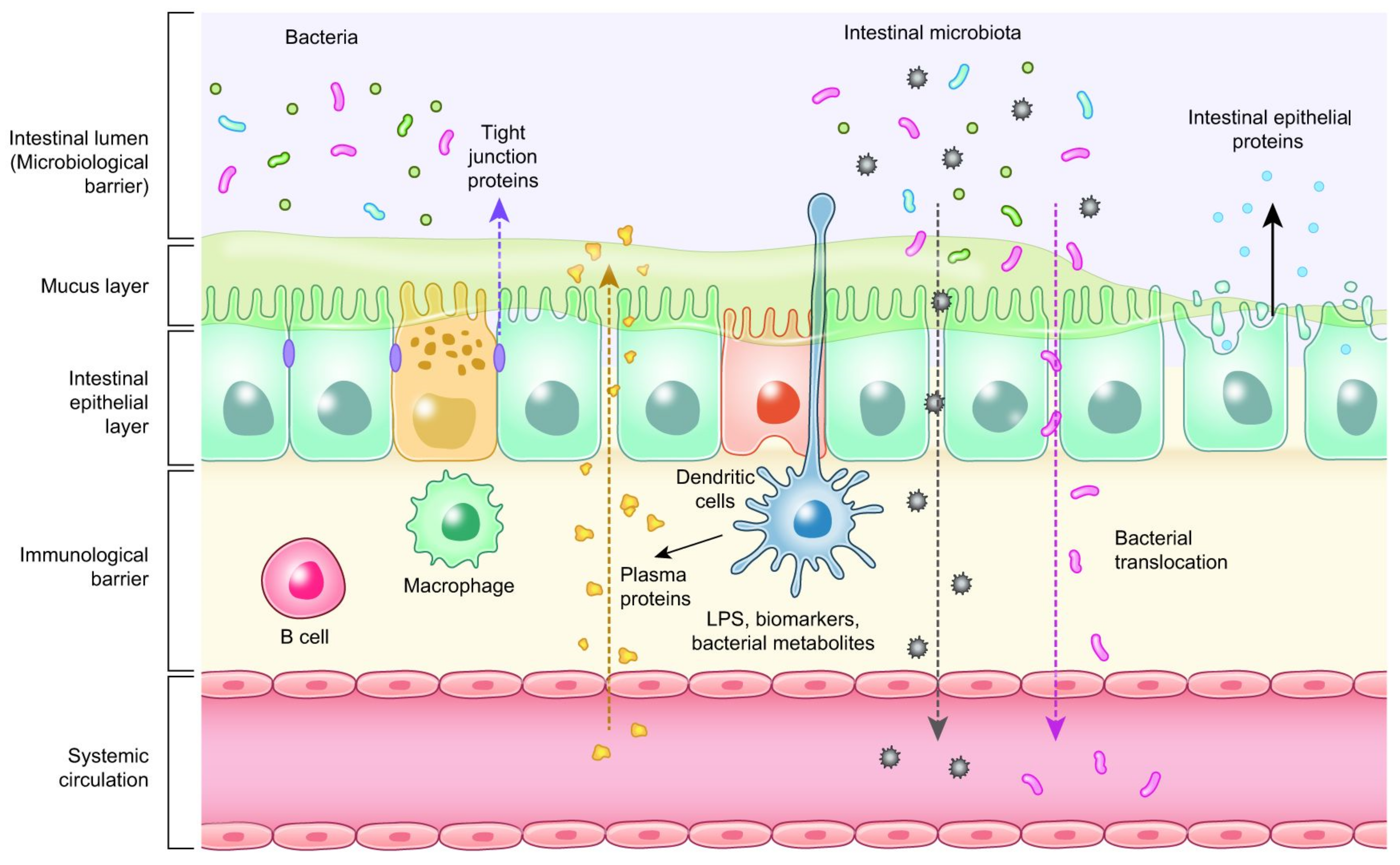
4. Biomarkers Related to Intestinal Health of Animals
| Measurement/Function | Biomarker Type |
|---|---|
| Antioxidant activity | Superoxide dismutase (SOD), Thiobarbituric acid reactive substances (TBARS), Total antioxidant capacity |
| Gene expression of host protein biomarkers and tight junction |
Fatty acid binding protein (FABP), Fibronectin, Occludin, Zonula occludens, Claudins |
| Immune activity | Acute phase proteins, Calprotectin, Lipocalin, Immunoglobulins (IgA), Interferon gamma (INF-γ) |
| Intestinal permeability | Fluorescein isothiocyanate dextran (FITC-d), Trans epithelial electrical resistance (TEER), Bacterial translocation |
| Enterocyte function | Extracellular signal-regulated kinase (ERK), Citrulline |
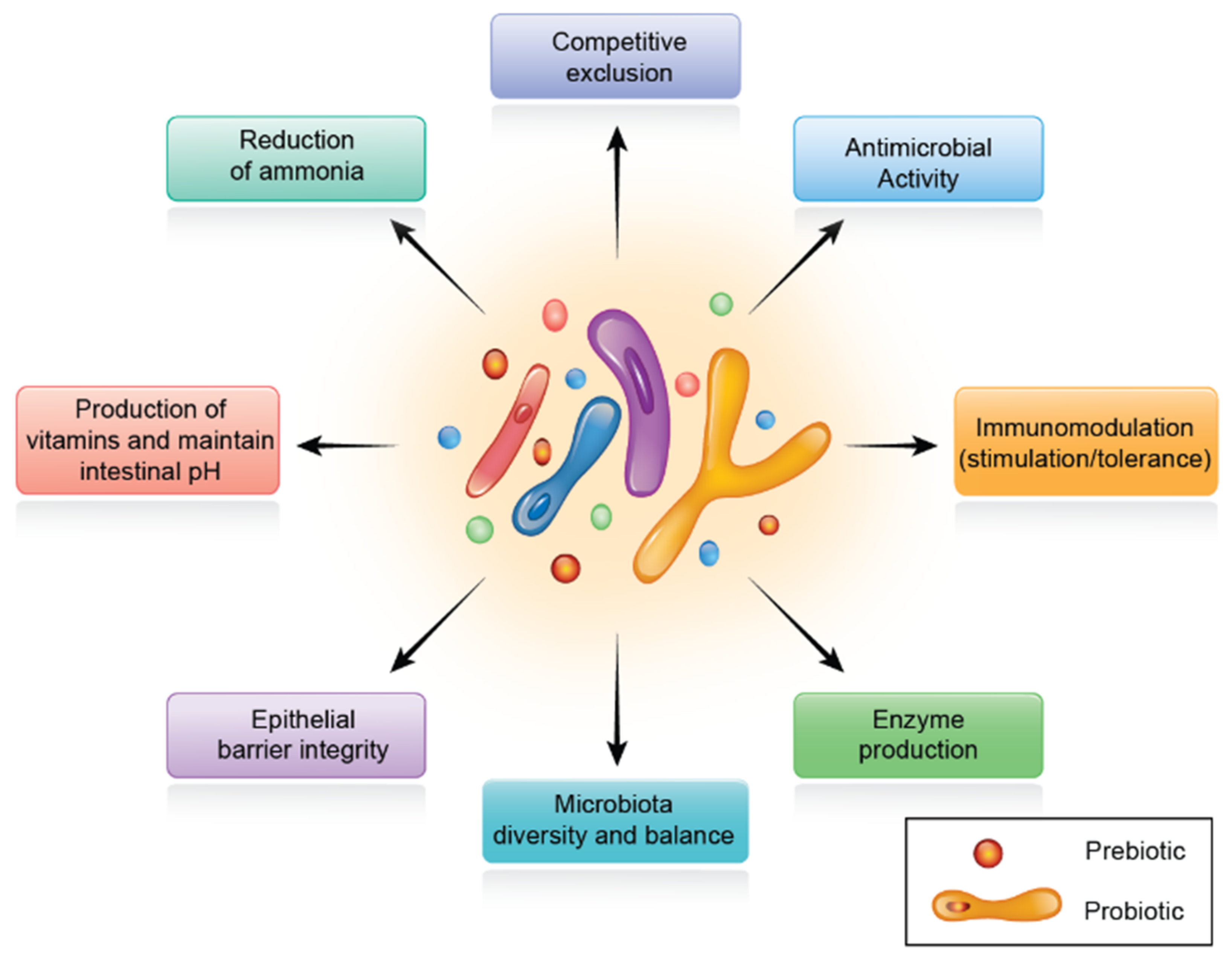
5. Probiotics
6. Prebiotics
7. Synbiotics
7.1. Role of Synbiotics in Poultry Production
7.2. The Role of Short Chain Fatty Acids (SCFAs) on Digestive Physiology
8. Phytogenic Feed Additives
| Enterotypes | Biological Activities | Favorable Substance/s |
|---|---|---|
| Bacteroides |
|
proteins and fats |
| Prevotella |
|
high fiber diet |
| Ruminococcus |
|
Sugars |
9. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms10020395
References
- BisBischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.-D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal Permeability—A New Target for Disease Prevention and Therapy. BMC Gastroenterol. 2014, 14, 189.
- Zoetendal, E.G.; Rajilic-Stojanovic, M.; de Vos, W.M. High-Throughput Diversity and Functionality Analysis of the Gastrointestinal Tract Microbiota. Gut 2008, 57, 1605–1615.
- Ackermann, W.; Coenen, M.; Schrödl, W.; Shehata, A.A.; Krüger, M. The Influence of Glyphosate on the Microbiota and Production of Botulinum Neurotoxin During Ruminal Fermentation. Curr. Microbiol. 2015, 70, 374–382.
- Schrödl, W.; Krüger, S.; Konstantinova-Müller, T.; Shehata, A.A.; Rulff, R.; Krüger, M. Possible Effects of Glyphosate on Mucorales Abundance in the Rumen of Dairy Cows in Germany. Curr. Microbiol. 2014, 69, 817–823.
- Krüger, M.; Neuhaus, J.; Herrenthey, A.G.; Gökce, M.M.; Schrödl, W.; Shehata, A.A. Chronic Botulism in a Saxony Dairy Farm: Sources, Predisposing Factors, Development of the Disease and Treatment Possibilities. Anaerobe 2014, 28, 220–225.
- Abuajamieh, M.; Kvidera, S.K.; Fernandez, M.V.S.; Nayeri, A.; Upah, N.C.; Nolan, E.A.; Lei, S.M.; DeFrain, J.M.; Green, H.B.; Schoenberg, K.M.; et al. Inflammatory Biomarkers Are Associated with Ketosis in Periparturient Holstein Cows. Res. Vet. Sci. 2016, 109, 81–85.
- López-García, P.; Eme, L.; Moreira, D. Symbiosis in Eukaryotic Evolution. J. Theor. Biol. 2017, 434, 20–33.
- Wren, B.W. Microbial Genome Analysis: Insights into Virulence, Host Adaptation and Evolution. Nat. Rev. Genet. 2000, 1, 30–39.
- Lee, S.; La, T.-M.; Lee, H.-J.; Choi, I.-S.; Song, C.-S.; Park, S.-Y.; Lee, J.-B.; Lee, S.-W. Characterization of Microbial Communities in the Chicken Oviduct and the Origin of Chicken Embryo Gut Microbiota. Sci. Rep. 2019, 9, 6838.
- Kikuchi, Y.; Hosokawa, T.; Nikoh, N.; Meng, X.-Y.; Kamagata, Y.; Fukatsu, T. Host-Symbiont Co-Speciation and Reductive Genome Evolution in Gut Symbiotic Bacteria of Acanthosomatid Stinkbugs. BMC Biol. 2009, 7, 2.
- Tellez, G. Prokaryotes Versus Eukaryotes: Who Is Hosting Whom? Front. Vet. Sci. 2014, 1, 3.
- Blaser, M.J. Who Are We? Indigenous Microbes and the Ecology of Human Diseases. EMBO Rep. 2006, 7, 956–960.
- Ulluwishewa, D.; Anderson, R.C.; McNabb, W.C.; Moughan, P.J.; Wells, J.M.; Roy, N.C. Regulation of Tight Junction Permeability by Intestinal Bacteria and Dietary Components. J. Nutr. 2011, 141, 769–776.
- Ballard, S.T.; Hunter, J.H.; Taylor, A.E. Regulation of Tight-Junction Permeability During Nutrient Absorption Across the Intestinal Epithelium. Annu. Rev. Nutr. 1995, 15, 35–55.
- González-Mariscal, L.; Tapia, R.; Chamorro, D. Crosstalk of Tight Junction Components with Signaling Pathways. Biochim. Biophys. Acta (BBA) Biomembr. 2008, 1778, 729–756.
- Harhaj, N.S.; Antonetti, D.A. Regulation of Tight Junctions and Loss of Barrier Function in Pathophysiology. Int. J. Biochem. Cell. Biol. 2004, 36, 1206–1237.
- Gilani, S.; Howarth, G.S.; Kitessa, S.M.; Tran, C.D.; Forder, R.E.A.; Hughes, R.J. New Biomarkers for Increased Intestinal Permeability Induced by Dextran Sodium Sulphate and Fasting in Chickens. J. Anim. Physiol. Anim. Nutr. 2017, 101, e237–e245.
- Ducatelle, R.; Goossens, E.; De Meyer, F.; Eeckhaut, V.; Antonissen, G.; Haesebrouck, F.; Van Immerseel, F. Biomarkers for Monitoring Intestinal Health in Poultry: Present Status and Future Perspectives. Vet. Res. 2018, 49, 43.
- Tellez, G.; Latorre, J.D.; Kuttappan, V.A.; Kogut, M.H.; Wolfenden, A.; Hernandez-Velasco, X.; Hargis, B.M.; Bottje, W.G.; Bielke, L.R.; Faulkner, O.B. Utilization of Rye as Energy Source Affects Bacterial Translocation, Intestinal Viscosity, Microbiota Composition, and Bone Mineralization in Broiler Chickens. Front. Genet. 2014, 5, 339.
- Chen, J.; Tellez, G.; Richards, J.D.; Escobar, J. Identification of Potential Biomarkers for Gut Barrier Failure in Broiler Chickens. Front. Vet. Sci. 2015, 2, 14.
- Ruff, J.; Tellez, G.; Forga, A.J.; Señas-Cuesta, R.; Vuong, C.N.; Greene, E.S.; Hernandez-Velasco, X.; Uribe, Á.J.; Martínez, B.C.; Angel-Isaza, J.A.; et al. Evaluation of Three Formulations of Essential Oils in Broiler Chickens under Cyclic Heat Stress. Animals 2021, 11, 1084.
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER Measurement Techniques for In Vitro Barrier Model Systems. J. Lab. Autom. 2015, 20, 107–126.
- Janssen Duijghuijsen, L.M.; Grefte, S.; de Boer, V.C.J.; Zeper, L.; van Dartel, D.A.M.; van der Stelt, I.; Bekkenkamp-Grovenstein, M.; van Norren, K.; Wichers, H.J.; Keijer, J. Mitochondrial ATP Depletion Disrupts Caco-2 Monolayer Integrity and Internalizes Claudin 7. Front. Physiol. 2017, 8, 794.
- Baxter, M.F.A.; Latorre, J.D.; Dridi, S.; Merino-Guzman, R.; Hernandez-Velasco, X.; Hargis, B.M.; Tellez-Isaias, G. Identification of Serum Biomarkers for Intestinal Integrity in a Broiler Chicken Malabsorption Model. Front. Vet. Sci. 2019, 6, 144.
- Windmueller, H.G.; Spaeth, A.E. Source and Fate of Circulating Citrulline. Am. J. Physiol. Endocrinol. Metab. 1981, 241, E473–E480.
- Berkeveld, M.; Langendijk, P.; Verheijden, J.H.M.; Taverne, M.A.M.; van Nes, A.; van Haard, P.; Koets, A.P. Citrulline and Intestinal Fatty Acid-Binding Protein: Longitudinal Markers of Postweaning Small Intestinal Function in Pigs? J. Anim. Sci. 2008, 86, 3440–3449.
- Iizuka, M. Wound Healing of Intestinal Epithelial Cells. WJG 2011, 17, 2161.
- Schrezenmeir, J.; de Vrese, M. Probiotics, Prebiotics, and Synbiotics-Approaching a Definition. Am J. Clin. Nutr. 2001, 73, 361S–364S.
- Yurong, Y.; Ruiping, S.; Shimin, Z.; Yibao, J. Effect of Probiotics on Intestinal Mucosal Immunity and Ultrastructure of Cecal Tonsils of Chickens. Arch. Anim. Nutr. 2005, 59, 237–246.
- Vanderpool, C.; Yan, F.; Polk, D.B. Mechanisms of Probiotic Action: Implications for Therapeutic Applications in Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2008, 14, 1585–1596.
- Prado-Rebolledo, O.F.; de Jesus Delgado-Machuca, J.; Macedo-Barragan, R.J.; Garcia-Márquez, L.J.; Morales-Barrera, J.E.; Latorre, J.D.; Hernandez-Velasco, X.; Tellez, G. Evaluation of a Selected Lactic Acid Bacteria-Based Probiotic on Salmonella enterica Serovar Enteritidis Colonization and Intestinal Permeability in Broiler Chickens. Avian Pathol. 2017, 46, 90–94.
- Molinaro, F.; Paschetta, E.; Cassader, M.; Gambino, R.; Musso, G. Probiotics, Prebiotics, Energy Balance, and Obesity: Mechanistic Insights and Therapeutic Implications. Gastroenterol. Clin. North Am. 2012, 41, 843–854.
- Pourabedin, M.; Zhao, X. Prebiotics and Gut Microbiota in Chickens. FEMS MicroBiol. Lett. 2015, 362, fnv122.
- Liu, X.; Cao, S.; Zhang, X. Modulation of Gut Microbiota-Brain Axis by Probiotics, Prebiotics, and Diet. J. Agric. Food Chem. 2015, 63, 7885–7895.
- Hamilton-Miller, J.M.T. Probiotics and Prebiotics in the Elderly. Postgrad. Med. J. 2004, 80, 447–451.
- Hedin, C.; Whelan, K.; Lindsay, J.O. Evidence for the Use of Probiotics and Prebiotics in Inflammatory Bowel Disease: A Review of Clinical Trials. Proc. Nutr. Soc. 2007, 66, 307–315.
- Everard, A.; Lazarevic, V.; Derrien, M.; Girard, M.; Muccioli, G.G.; Muccioli, G.M.; Neyrinck, A.M.; Possemiers, S.; Van Holle, A.; François, P.; et al. Responses of Gut Microbiota and Glucose and Lipid Metabolism to Prebiotics in Genetic Obese and Diet-Induced Leptin-Resistant Mice. Diabetes 2011, 60, 2775–2786.
- Ducatelle, R.; Eeckhaut, V.; Haesebrouck, F.; Van Immerseel, F. A Review on Prebiotics and Probiotics for the Control of Dysbiosis: Present Status and Future Perspectives. Animal 2015, 9, 43–48.
- Ajuwon, K.M. Toward a Better Understanding of Mechanisms of Probiotics and Prebiotics Action in Poultry Species. J. Appl. Poult. Res. 2016, 25, 277–283.
- Awad, W.A.; Ghareeb, K.; Abdel-Raheem, S.; Böhm, J. Effects of Dietary Inclusion of Probiotic and Synbiotic on Growth Performance, Organ Weights, and Intestinal Histomorphology of Broiler Chickens. Poult. Sci. 2009, 88, 49–56.
- Maiorano, G.; Sobolewska, A.; Cianciullo, D.; Walasik, K.; Elminowska-Wenda, G.; Slawinska, A.; Tavaniello, S.; Zylinska, J.; Bardowski, J.; Bednarczyk, M. Influence of in Ovo Prebiotic and Synbiotic Administration on Meat Quality of Broiler Chickens. Poult. Sci. 2012, 91, 2963–2969.
- Yang, X.J.; Li, W.L.; Feng, Y.; Yao, J.H. Effects of Immune Stress on Growth Performance, Immunity, and Cecal Microflora in Chickens. Poult. Sci. 2011, 90, 2740–2746.
- EC (European Commission). Regulation 1831 of the European Parliament and of the Council of 22 September 2003 on Additives for Use in Animal Nutrition. Off. J. Eur. Union L. 2003, 268, 29–43.
- Giannenas, I.; Florou-Paneri, P.; Papazahariadou, M.; Christaki, E.; Botsoglou, N.A.; Spais, A.B. Effect of Dietary Supplementation with Oregano Essential Oil on Performance of Broilers after Experimental Infection with Eimeria tenella. Arch. Anim. Nutr. 2003, 57, 99–106.
- Isabel, B.; Santos, Y. Effects of Dietary Organic Acids and Essential Oils on Growth Performance and Carcass Characteristics of Broiler Chickens. J. Appl. Poult. Res. 2009, 18, 472–476.
- Jamroz, D.; Wiliczkiewicz, A.; Wertelecki, T.; Orda, J.; Skorupińska, J. Use of Active Substances of Plant Origin in Chicken Diets Based on Maize and Locally Grown Cereals. Br. Poult. Sci. 2005, 46, 485–493.
- McReynolds, J.; Waneck, C.; Byrd, J.; Genovese, K.; Duke, S.; Nisbet, D. Efficacy of Multistrain Direct-Fed Microbial and Phytogenetic Products in Reducing Necrotic Enteritis in Commercial Broilers. Poult. Sci. 2009, 88, 2075–2080.
- Murugesan, G.R.; Syed, B.; Haldar, S.; Pender, C. Phytogenic Feed Additives as an Alternative to Antibiotic Growth Promoters in Broiler Chickens. Front. Vet. Sci. 2015, 2, 21.
- Yener, Y.; Yalçin, S.; Çolpan, İ. Effects of Dietary Supplementation of Red Ginseng Root Powder on Performance, Immune System, Cecal Microbial Population and Some Blood Parameters in Broilers. Ank. Üniv. Vet. Fakültesi Derg. 2020, 68, 137–145.
- Jha, R.; Berrocoso, J.D. Review: Dietary Fiber Utilization and Its Effects on Physiological Functions and Gut Health of Swine. Animal 2015, 9, 1441–1452.
- Schokker, D.; Jansman, A.J.M.; Veninga, G.; de Bruin, N.; Vastenhouw, S.A.; de Bree, F.M.; Bossers, A.; Rebel, J.M.J.; Smits, M.A. Perturbation of Microbiota in One-Day Old Broiler Chickens with Antibiotic for 24 Hours Negatively Affects Intestinal Immune Development. BMC Genom. 2017, 18, 241.
- Adak, A.; Khan, M.R. An Insight into Gut Microbiota and Its Functionalities. Cell Mol. Life Sci. 2019, 76, 473–493.
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.-M.; et al. Enterotypes of the Human Gut Microbiome. Nature 2011, 473, 174–180.
