With depression becoming increasingly prevalent, being closely associated with stress, and many patients exhibiting resistance to current treatments, depression pathophysiology requires further elucidation [1][2][3][4]. Recent research has shown complex bidirectional links between the brain and the gut, and the gut microbiota and the influence of diet is beginning to provide new clues to the complex nature of this disorder. It is well known that diet is a key modulator of gut microbial composition. In humans, good quality plant-based diets such as the Mediterranean diet have been shown to reduce pathogenic bacteria in the gut, increase Bifidobacterium and Clostridium, as well as lower the risk of depression, while poorer quality diets such as the Western diet have been shown to reduce Lactobacillus in the gut, reduce overall gut microbial diversity and have been associated with increased depression risk [5][6]. Evaluating the effects of diets on the brain-to-gut and gut-to-brain mechanisms in animal models of stress and depression may aid in the elucidation of the pathophysiology of depression and may provide novel therapeutic approaches. Thus, we will review the effects of probiotic and plant-based prebiotic supplementation in animal models of stress and depression, particularly in studies which evaluated relevant alterations discussed earlier in this review in both the gut and the brain to better appreciate the bidirectional lines of communication involved. For further information about animal models of stress and depression, we refer readers to our published review on the Effects of Stress and Diet on the “Brain-Gut” and “Gut-Brain” Pathways in Animal Models of Stress and Depression (DOI: 10.3390/ijms23042013). To our knowledge, studies under this scope which utilized diets or supplements mimicking a westernised diet are lacking, thus we will conclude with a brief summary of key findings of interest for such diets.
- animal
- depression
- diet
- gut–brain axis
- microbiota
- pathways
- prebiotics
1. Prebiotics and Probiotics Affect the Pathways along the Gut–brain Axis
Neuroplasticity, involving the reorganisation of synaptic connections, is well known to be disrupted in depression and is largely modulated by serotonin, through synapse formation [2][7]. Abnormal serotonin signalling in brain regions such as the hippocampus and prefrontal cortex are known to be major pathophysiological mechanisms of depression. Depressive patients are frequently reported to have decreased serotonin metabolites and common antidepressants such as serotonin reuptake inhibitors have been demonstrated to increase serotonin and brain-derived neurotrophic factor (BDNF) in the brain [2][8].
According to Bear et al. [5], healthier diets rich in fruit and vegetables typically contain higher amounts of prebiotics such as polyphenols and prebiotic soluble fibres including fructooligosaccharides and galactooligosaccharides and are associated with decreased risk of depression. Widely studied for their anti-inflammatory and antioxidant effects, polyphenols are known to modulate the gut microbiota and, as a dietary compound, are known to be largely indigestible without biotransformation by the colonic microbiota [9]. Modulation of the gut microbiota leads to widespread effects along the gut-brain pathways (Figure 1).
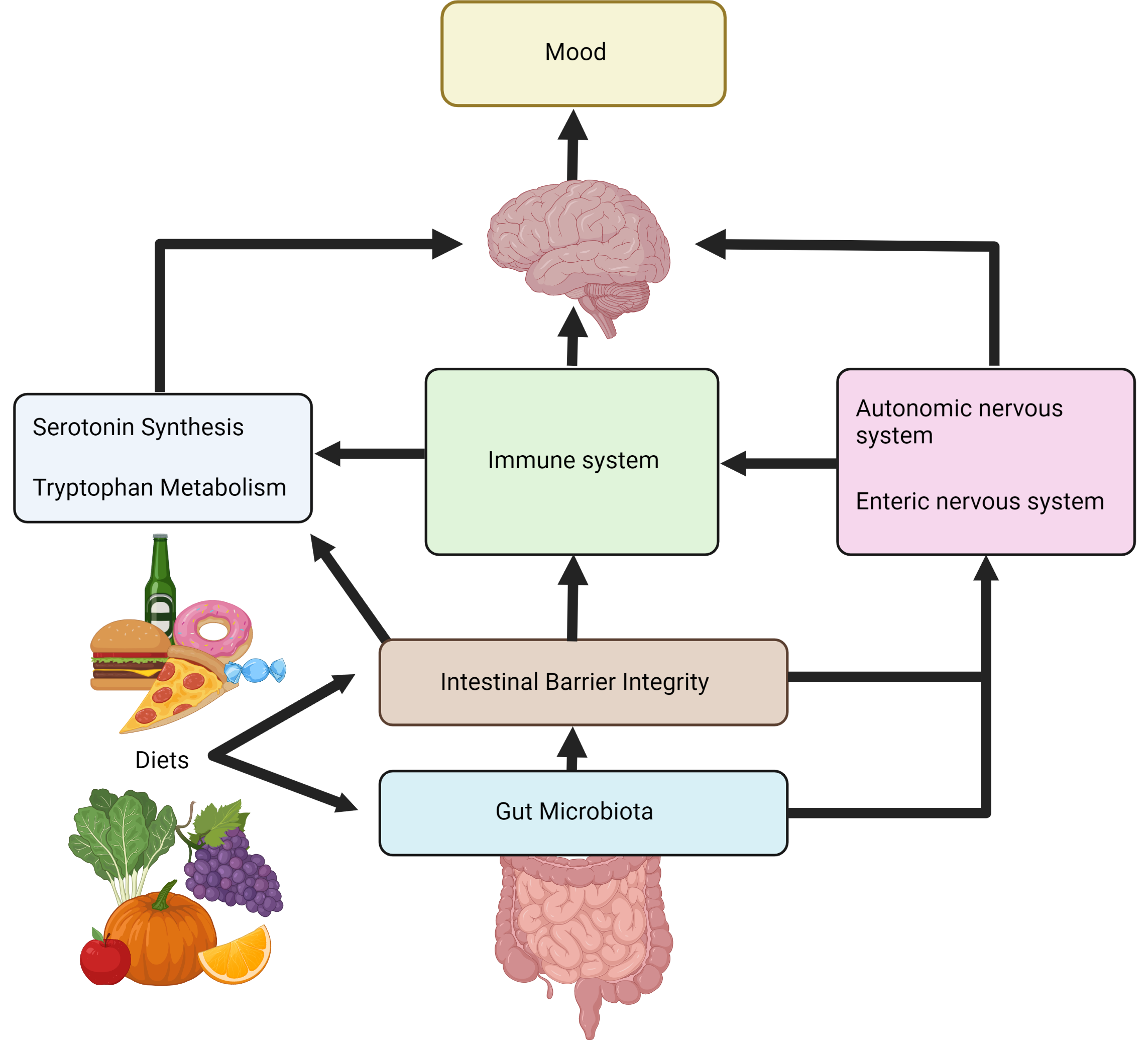
Figure 1. An overview of the major gut-brain pathways affected by diet*.
Observational studies in humans have shown an inverse association between polyphenol intake and depression risk [10]. Such effects may be due to increased Bifidobacterium and Lactobacillus abundance since polyphenols are known to modulate the gut microbiota by increasing these bacterial strains [11]. In animal models of stress and depression, supplementation with Bifidobacterium and Lactobacillus appeared to have an effect on colonic serotonin levels in mice, and possibly the availability of its precursor tryptophan, since it increased levels of colonic tryptophan hydroxylase 1 (TPH1) and the intermediate 5-hydroxytryptophan (5-HTP), previously decreased by chronic unpredictable mild stress (CUMS) [12][13]. Tian et al. [14] did not report on colonic serotonin levels, but showed Bifidobacterium longum supplementation increased levels of serum 5-HTP, consistent with increased serum serotonin levels found by Oh et al. [12] following L. rhamnosus supplementation, suggesting downregulation of the kynurenine pathway, which modulates the availability of tryptophan required for serotonin synthesis, in the colon and periphery [15] (Table 1). Since the kynurenine pathway is disturbed in depression, affecting serotonin signalling in the brain, Bifidobacteria and Lactobacilli may restore serotonergic signalling and potentially alleviate depression by counteracting these effects [2][7].
Table 1. The effects of diet on gut pathology, behaviour and potential mechanisms for the brain in animal models of stress and depression.
|
Model, Duration and Species |
Diet/Treatment |
Gut Pathology |
Behaviour |
Possible Gut–brain Pathways |
Authors |
|
Chronic unpredictable mild stress (CUMS)—4 weeks Male Wistar rats |
Standard diet + Orally gavaged fructo-oligosaccharides (FOS)/ galacto-oligosaccharides (GOS) and probiotics (Bifidobacterium longum and Lactobacillus rhamnosus) |
· Colonic serotonin (5-HT) and tryptophan hydroxylase 1 (TPH1); Attenuated by FOS/GOS and probiotics |
· Depressive-like behaviour; Attenuated by FOS/GOS and probiotics · Anhedonia; Attenuated by FOS/GOS and probiotics |
Enteroendocrine alterations and perturbations in tryptophan metabolism |
[16] |
|
CUMS—4 weeks C57BL6 mice of unspecified sex |
Standard diet + orally gavaged Bifidobacterium longum subspecies infantis strain CCFM687 in 10% skimmed milk solution at 109 CFU/mL daily for 6 weeks |
· Total short chain fatty acids (SCFA); Attenuated by CCFM687 |
· Depressive-like behaviour; Attenuated by CCFM687 · Anxiety-like behaviour; Attenuated by CCFM687 |
Alterations to SCFA regulation of intestinal permeability affected systemic inflammation and HPA axis function, leading to changes in neuroplasticity in the frontal cortex |
[14] |
|
CUMS—4 weeks Male Sprague Dawley rats |
Standard diet + orally gavaged Fluoxetine (1.82 mg/kg), green tea (64.8 mg/kg) or jasmine tea (21.6 mg/kg, 64.8 mg/kg and 194.4 mg/kg) |
· Colonic structural integrity (inflammatory infiltration, decreased goblet cell number and shallow crypts); Attenuated by jasmine tea |
· Depressive-like behaviour; Attenuated by Fluoxetine, green tea and jasmine tea
|
Alterations in peripheral (glucagon-like peptide 1) GLP-1 release in the gut with subsequent alterations in vagal-dependent central GLP-1 signalling in the brain |
[17] |
|
CUMS—4 weeks Male C57BL6 mice |
Standard diet + orally gavaged saline, partially hydrolysed guar gum (PHGG) (600 mg/kg)), Fluoxetine (0.5 mg/kg) and PHGG (600 mg/kg) or Fluoxetine (1.0 mg/kg) |
· Faecal SCFAs (lactic acid, acetic acid and valeric acid); Attenuated by PHGG, PHGG + Fluoxetine and Fluoxetine |
· Depressive-like behaviour; Attenuated by PHGG, PHGG + Fluoxetine and Fluoxetine |
Alterations to SCFA regulation of intestinal permeability leading to changes in serotonergic and dopaminergic neurotransmission in the striatum and hippocampus |
[18] |
|
CUMS—5 weeks Male C57BL6 mice |
Standard diet + orally gavaged CCFM105 Bifidobacterium breve (0.1 mL/10 g body weight) |
· Colonic serotonin (5-HT) and tryptophan hydroxylase 1 (THP1); Attenuated by B.breve · Colonic SCFAs (propionate, butyrate, isobutyric acid and isovaleric acid); All attenuated by B. breve
|
· Anhedonia; Attenuated by B. breve · Depressive-like behaviour; Attenuated by B. breve · Anxiety-like behaviour; Attenuated by B.breve |
Alterations to SCFA regulation of intestinal permeability and colonic enzymes involved in serotonin synthesis affected 5-hydroxytryptophan (5-HTP) levels. 5-HTP is capable of crossing the BBB, thus these alterations influenced neuroplasticity in the hippocampus. Alterations in HPA axis function may have also contributed the changes in brain and behaviour |
[13] |
|
CUMS—7 weeks Male C57BL6 mice |
Standard diet or standard diet + glycated milk casein (Gc) or glycated milk casein fermented with Lactobacillus rhamnosus (FGc) |
· Colonic tryptophan hydroxylase 1 (TPH1) and free fatty acid receptor 2 (GPR43); Attenuated by FGc · Colonic inflammation (iNOS and COX-2); iNOS attenuated by FGc and COX-2 attenuated by Gc and FGc · Colonic barrier integrity (Zo-1, Cldn-5 and occludin); Attenuated by Gc and FGc bar occludin |
· Anxiety-like behaviour; Attenuated by FGc |
Alterations in colonic inflammation may modulate colitis pathology, affecting intestinal permeability and this may affect the transport of gut-derived molecules such as inflammatory mediators in circulation which can reach the brain. These alterations, which may also involve the HPA axis, may affect neuroplasticity in the brain |
[12] |
|
Chronic restraint stress (CRS)—1 week Male C57BL6 mice |
Standard diet + orally gavaged saline or Lactobacillus johnsonii |
· Jejunal and ileal barrier integrity (Zo-1, Cldn-1 and occludin); Attenuated by L. johnsonii · Ileal inflammation (TNF- α and INF-γ); Attenuated by L. johnsonii |
· Memory; Attenuated by L. johnsonii |
Alterations in small bowel inflammation may modulate intestinal barrier integrity subsequently impacting hippocampal dopaminergic, serotonergic and GABAergic neurotransmission |
[19] |
|
CRS—2 weeks Male Swiss mice |
Standard diet + orally gavaged Bifidobacterium longum BG0014, Bifidobacterium longum ssp. infantis Bl11471, Bifidobacterium animalis BL0005, Bifidobacterium animalis ssp. lactis 420, Lactobacillus paracasei Lpc-37, Lactobacillus salivarius Ls-33, Lactobacillus plantarum LP12418, Lactobacillus plantarum LP12151, Lactobacillus plantarum LP12407, Lactobacillus acidophilus LA11873, Lactobacillus rhamnosus LX11881 or Lactobacillus helveticus LH0138 (1 × 109 CFU/day for 5 weeks) |
· No significant changes to mRNA expression; No significant effects of probiotics |
· Anxiety-like behaviour; Attenuated by Lpc-37, LP12407 and LP12418 · Depressive-like behaviour; Attenuated by Lpc-37, LP12407 and LP12418 · Memory; Attenuated Lpc-37, LP12407 and LP12418 |
Alterations in behaviour modulated by changes in gut microbiota with impacts on GABAergic neurotransmission in the prefrontal cortex requiring further study |
[20] |
|
Chronic social defeat stress (CSDS)—10 days Male C57BL6 mice and male CD1 mice |
Standard diet + Clostridium butyricum MIYAIRI 588 (>5 × 106/CFU) in drinking water for 4 weeks |
· Colonic inflammation (TNF-α, IL-1β and IL-6); MIYAIRI 588 attenuated all · Colonic barrier integrity (Zo-1); Attenuated by MIYAIRI 588 · Colonic free fatty acid receptors 2 and3; Attenuated by MIYAIRI 588 |
· Depressive-like behaviour; Attenuated by MIYAIRI 588 · Social interaction; Attenuated by MIYAIRI 588 |
Alterations in neuroinflammation and colonic inflammation modulated by alterations in the gut microbiota and intestinal barrier integrity |
[21] |
CUMS—chronic unpredictable mild stress; CRS—chronic restraint stress; MS—maternal separation; CORT—chronic corticosteroid administration; CSDS—chronic social defeat stress; LH—learned helplessness; HPA—hypothalamic-pituitary-adrenal axis; BBB—blood brain barrier.
Probiotic and prebiotic supplementation appear to have consistent effects on the brain and behaviour. In the studies reviewed, dietary supplementation with fructooligosaccharides and galactooligosaccharides, Bifidobacteria or Lactobacilli attenuated depressive- and anxiety-like behaviours in rats and mice [12][13][14][16][20][22][23][24]. Oh et al. [12] and Tian et al. [13] reported that supplementation with these probiotics increased hippocampal levels of the serotonin receptor, 5HT1a. Increased serotonin levels in the frontal cortex were also consistently reported as a result of probiotic or prebiotic supplementation [14][16]. Jasmine tea, considered a prebiotic has also been demonstrated to restore frontal cortex and hippocampal serotonin in a similar manner to fluoxetine in association with restoration of the Firmicutes:Bacteroidetes ratio [17]. BDNF, in addition to being neuroprotective, shares close molecular connections with serotonin receptors, and thus also influences neuroplasticity[8]. Supplementation with B. longum, B. breve and Clostridium butyricum all reversed decreases in hippocampal and frontal cortex BDNF expression caused by CUMS. Lactobacillus probiotics administered in animal models of depression were also able to restore BDNF levels in the frontal cortex in maternal separation (MS) and the hippocampus in chronic restraint stress (CRS) [19][24]. Therefore, the effects of prebiotics such as fructooligosaccharides and galactooligosaccharides, or probiotics such as Lactobacillus or Bifidobacterium, on behaviour in these animal studies may be a result of the restoration of neuroplasticity brought about by increased serotonin and BDNF levels in key brain regions involved in depression pathophysiology, however colonic serotonin modulation by the gut microbiota appears to be host species-dependent and separate to the central nervous system, while the mechanism behind the modulation of central nervous system BDNF levels by the gut microbiota remains to be elucidated.
2. Diet, Stress and Intestinal Barrier Integrity
Depressive patients are frequently reported to have comorbid inflammatory bowel diseases and stress and depression are known to be associated with low-grade colonic inflammation, possibly due to gut microbiota dysbiosis in association with disrupted intestinal barrier integrity [5][25]. Dysbiosis of the gut microbiota may lead to alterations in the levels of Toll-like receptor (TLR) signalling in the gut, inducing the intestinal inflammatory response [12]. This inflammatory response weakens the tight junction proteins, which function to prevent leakage of solutes across the gut epithelium, compromising intestinal barrier integrity [26]. Disrupted intestinal barrier integrity, also affected by hypothalamic-pituitary-adrenal (HPA) axis dysfunction may also place the brain and gut at risk since immune signalling and the inflammatory response are also initiated both locally and systemically after the microbiota or the outer surface membrane of Gram-negative gut bacteria known as lipopolysaccharide endotoxin (LPS) are exposed to intestinal epithelial cells and associated resident immune cells in the lamina propria, inducing TLR-signalling, which activates inflammatory pathways (see the published review for further detail) [12][27]. Probiotics such as Bifidobacteria and/or Lactobacilli may reduce dysregulation of the HPA axis brought about by chronic stress, and in turn regulate gut health. Changes in the composition of the microbiota have been attributed to overactivation of the HPA axis and overproduction of corticosterone [28], leading to increased intestinal permeability, known as the rate at which gut luminal contents such as the gut microbiota and/or its products cross the gut epithelium [29][30][31]. Gut permeability is also upregulated by a higher Firmicutes:Bacteroidetes ratio [32] and downregulated by Bifidobacteria [33]. In animal models which utilised CUMS, increased hypothalamic corticotropin-releasing factor (CRF) and serum corticosterone levels were attenuated by Bifidobacterium and Lactobacillus supplementation [12][13][14][19]. Bifidobacterium supplementation was also demonstrated to reverse a CUMS-induced increase in circulating tumor necrosis factor (TNF-α), a potent pro-inflammatory mediator, possibly due to a reduction in the exposure of LPS or the gut although the mechanism of LPS reduction is unclear [13]. A possible explanation may be alterations in the expression of TLRs at the level of the paraventricular nucleus of the hypothalamus. Murray et al. [34] showed that administration of the probiotic kefir to mice exposed to LPS reduced TLR4 expression in the paraventricular nucleus in association with reduced anxiety-like behaviour and stress activity in male mice, however probiotic kefir only reduced depressive-like behaviour in females. These effects resulted in reduced central and peripheral inflammatory cytokines in a sex-specific manner [34]. These restorative effects on the HPA axis and inflammation may therefore have similar effects on gut health due to the close association between stress and poor gut health.
While stress had a clear effect on intestinal barrier integrity in the animal studies reviewed since tight junction proteins were decreased in stressed mice exposed to CUMS, CRS and chronic social defeat stress (CSDS) [12][14][19][25][35], the studies by Oh et al. [12] and Tian et al. [21] showed that decreased colonic barrier integrity brought about by stress could be abolished by supplementation with Lactobacillus and Clostridium probiotics, respectively, demonstrating that intestinal permeability may not only be under regulation by stress. Wang et al. [19] showed similar effects for the jejunum and ileum with Lactobacillus in CRS-treated mice. These effects found on intestinal barrier integrity may involve anti-inflammatory mechanisms since the study by Oh et al. [12] showed that increased intestinal barrier integrity brought about by Lactobacillus was accompanied by colonic and neuronal suppression of both the pro-inflammatory mediators, inducible nitric oxide synthase and cyclooxygenase-2. Wang et al. [19] demonstrated that Lactobacillus could attenuate CRS-induced increases in ileal proinflammatory mediators TNF-α and interferon-γ (IFN) and increase the anti-inflammatory cytokine interleukin-10 (IL-10). Similarly, Clostridium supplementation attenuated CSDS-induced increases in colonic inflammation and hippocampal microglial activation.
Since the integrity of the intestinal barrier is associated with levels of short-chain fatty acids (SCFA), produced by the digestion of dietary fibre by the gut microbiota, probiotics may strengthen intestinal barrier activity via SCFA production in the gut. Based on the studies reviewed, SCFA production may be species-dependent since supplementation with B. breve, unlike B. longum, restored colonic SCFA levels previously decreased by CUMS [13]. However, probiotic supplementation with Lactobacillus or Clostridium was shown to restore expression of colonic free fatty acid receptors, previously decreased by chronic stress, suggesting restoration of the colon’s capacity to respond to SCFAs [12][21]. Overall, these effects may have strengthened the intestinal barrier, preventing exposure of the circulation to bacterial ligands such as LPS, thus preventing TLR-mediated neuroinflammation and depressive-like behaviour. Indeed, Burokas et al. [22] showed in their study that general increases in SCFA levels brought about by fructooligosaccharide and galactooligosaccharide supplementation were strongly correlated with positive changes in anhedonic (the reduced ability to experience pleasure), depression-like and social behaviour. Prebiotics such as guar gum may also beneficially shift the SCFA profile of the gut which has been shown to alleviate depressive-like behaviour by maintaining striatal and hippocampal serotonergic and dopaminergic neurotransmission; however, how SCFAs mediate these effects in the brain requires further investigation [36].
Contrary to the positive effects of probiotics and plant-based prebiotics on the gut–brain axis, the effects of diets which resemble a westernised diet such as high-fat diets or cafeteria diets on the pathways discussed remain to be elucidated. Current literature shows such westernised diets impair memory, alter neuroplasticity in key brain regions involved in depression, and increase neuroinflammation [37][38][39]. However, whether these effects modulate stress-related brain-to-gut and gut-to-brain mechanisms is unclear since studies evaluating brain and behaviour in conjunction with gut health in well-established animal models of stress and depression are lacking. de Sousa Rodrigues et al. [40] showed that stressed mice fed a high fat, high fructose-diet had increased anhedonia and anxiety-like behaviour in association with increased gut permeability and circulating TNF-α and IL-6 levels compared to their non-stressed counterparts. Yet, hippocampal cytokine expression was unaffected by both diet and stress, however this may have been related to the predatory stress model used [40]. de Sousa Rodrigues et al. [40] suggested that the increased gut permeability may be driven by high fat intake activating intestinal mast cells, leading to increased inflammatory cytokine production which may have promoted gut microbial dysbiosis, although the gut microbiota was not assessed in their study. Nonetheless, according to a review by Rohr et al. [41], high-fat diets increase intestinal permeability and local TLR-mediated inflammation, diminish the mucus layer of the gut and alter the composition of the gut microbiota. Further studies have shown that rodents fed high fat or westernised diets have decreased gut microbial diversity, suggesting that such diets may indeed have deleterious effects on the bidirectional mechanisms between the gut and the brain [42][43][44]. Interestingly, the composition of the gut microbiota in unstressed female mice fed a high fat diet has been shown to resemble that of female mice exposed to CUMS, but fed a standard diet; however, the same effect is not observed in males, thus females may be more susceptible to the potential negative effects of high fat diets on the gut–brain axis [42]. Hatton-Jones et al. [45] showed a Western diet decreased colonic tight junction protein expression in male mice, but this did not result in increased depressive-like behaviour and these effects were not synergistic with those of CRS. Yet, Ranyah Shaker et al. [46] demonstrated male Wistar rats fed a high-fat diet had increased dopamine and glutamate, but decreased serotonin in the brain, with increased circulating IL-6 in association with decreased Clostridium and Bacteroides abundance. In support of this, Saiyasit et al. [47] showed that Wistar rats fed a high-fat diet exhibited gut microbial dysbiosis after 2 weeks, which was followed by decreased hippocampal plasticity and dendritic spine density in association with cognitive decline, suggesting dysbiosis may drive brain pathology. Thus, westernised diets in rodents appear to have negative consequences for brain health possibly driven by gut-to-brain pathways, however the relevance of these changes in the context of stress and depression are unclear, warranting further study of westernised diets in common animal models of stress and depression.
3. Summary
- Prebiotics and probiotics show promise as dietary supplements which may alleviate depression pathophysiology
- Bifidobacteria and/or Lactobacilli supplementation restore neuroplasticity in key brain regions involved in depression
- Bifidobacteria and/or Lactobacilli supplementation restore intestinal barrier integrity and negative feedback in the hypothalamic-pituitary-adrenal (HPA) axis
- Westernised diets impair neuroplasticity in key brain regions involved in depression
- Westernised diets impair intestinal barrier integrity and the mucus layer of the gut
*Figure 1 created with BioRender.com
This entry is adapted from the peer-reviewed paper 10.3390/ijms23042013
References
- Czéh, B.; Fuchs, E.; Wiborg, O.; Simon, M. Animal models of major depression and their clinical implications. Progress in Neuro-Psychopharmacology and Biological Psychiatry 2016, 64, 293-310.https://doi.org/10.1016/j.pnpbp.2015.04.004
- Dean, J.; Keshavan, M. The neurobiology of depression: An integrated view. Asian journal of psychiatry 2017, 27, 101-111.10.1016/j.ajp.2017.01.025
- Hidaka, B.H. Depression as a disease of modernity: Explanations for increasing prevalence. Journal of Affective Disorders 2012, 140, 205-214.10.1016/j.jad.2011.12.036
- Milaneschi, Y.; Lamers, F.; Berk, M.; Penninx, B.W.J.B.P. Depression heterogeneity and its biological underpinnings: Toward immunometabolic depression. Biol. Psychiatry 2020, 88, 369-380.10.1016/j.biopsych.2020.01.014
- Bear, T.L.; Dalziel, J.E.; Coad, J.; Roy, N.C.; Butts, C.A.; Gopal, P.K. The role of the gut microbiota in dietary interventions for depression and anxiety. Advances in Nutrition 2020, 11, 890-907.10.1093/advances/nmaa016
- Taylor, A.M.; Holscher, H.D. A review of dietary and microbial connections to depression, anxiety, and stress. Nutritional neuroscience 2020, 23, 237-250.10.1080/1028415X.2018.1493808
- Wang, Q.; Timberlake, M.A.; Prall, K.; Dwivedi, Y. The recent progress in animal models of depression. Progress in Neuropsychopharmacology & Biological Psychiatry 2017, 77, 99-109.10.1016/j.pnpbp.2017.04.008
- Kraus, C.; Castrén, E.; Kasper, S.; Lanzenberger, R. Serotonin and neuroplasticity – links between molecular, functional and structural pathophysiology in depression. Neuroscience & Biobehavioral Reviews 2017, 77, 317-326.https://doi.org/10.1016/j.neubiorev.2017.03.007
- Caracci, F.; Harary, J.; Simkovic, S.; Pasinetti, G.M. Grape-derived polyphenols ameliorate stress-induced depression by regulating synaptic plasticity. Journal of Agricultural and Food Chemistry 2020, 68, 1808-1815.10.1021/acs.jafc.9b01970
- Firth, J.; Marx, W.; Dash, S.; Carney, R.; Teasdale, S.B.; Solmi, M.; Stubbs, B.; Schuch, F.B.; Carvalho, A.F.; Jacka, F., et al. The effects of dietary improvement on symptoms of depression and anxiety: A meta-analysis of randomized controlled trials. Psychosom Med 2019, 81, 265-280.10.1097/PSY.0000000000000673
- Filosa, S.; Di Meo, F.; Crispi, S. Polyphenols-gut microbiota interplay and brain neuromodulation. Neural Regen Res 2018, 13, 2055-2059.10.4103/1673-5374.241429
- Oh, N.S.; Joung, J.Y.; Lee, J.Y.; Song, J.G.; Oh, S.; Kim, Y.; Kim, H.W.; Kim, S.H. Glycated milk protein fermented with lactobacillus rhamnosus ameliorates the cognitive health of mice under mild-stress condition. Gut microbes 2020, 11, 1643-1661.10.1080/19490976.2020.1756690
- Tian, P.; O'Riordan, K.J.; Lee, Y.-k.; Wang, G.; Zhao, J.; Zhang, H.; Cryan, J.F.; Chen, W. Towards a psychobiotic therapy for depression: Bifidobacterium breve ccfm1025 reverses chronic stress-induced depressive symptoms and gut microbial abnormalities in mice. Neurobiology of Stress 2020, 12, 100216.https://doi.org/10.1016/j.ynstr.2020.100216
- Tian, P.; Zou, R.; Song, L.; Zhang, X.; Jiang, B.; Wang, G.; Lee, Y.-k.; Zhao, J.; Zhang, H.; Chen, W. Ingestion of bifidobacterium longum subspecies infantis strain ccfm687 regulated emotional behavior and the central bdnf pathway in chronic stress-induced depressive mice through reshaping the gut microbiota. Food & Function 2019, 10, 7588-7598.10.1039/c9fo01630a
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 2017, 112, 399-412.https://doi.org/10.1016/j.neuropharm.2016.07.002
- Li, H.; Wang, P.; Huang, L.; Li, P.; Zhang, D. Effects of regulating gut microbiota on the serotonin metabolism in the chronic unpredictable mild stress rat model. Neurogastroenterology & Motility 2019, 31, n/a-n/a.10.1111/nmo.13677
- Zhang, Y.; Huang, J.; Xiong, Y.; Zhang, X.; Lin, Y.; Liu, Z. Jasmine tea attenuates chronic unpredictable mild stress-induced depressive-like behavior in rats via the gut-brain axis. Nutrients 2021, 14, 99.10.3390/nu14010099
- Chen, Y.-h.; Xue, F.; Yu, S.-f.; Li, X.-s.; Liu, L.; Jia, Y.-y.; Yan, W.-j.; Tan, Q.-r.; Wang, H.-n.; Peng, Z.-w. Gut microbiota dysbiosis in depressed women: The association of symptom severity and microbiota function. Journal of Affective Disorders 2021, 282, 391-400.https://doi.org/10.1016/j.jad.2020.12.143
- Wang, H.; He, S.; Xin, J.; Zhang, T.; Sun, N.; Li, L.; Ni, X.; Zeng, D.; Ma, H.; Bai, Y. Psychoactive effects of lactobacillus johnsonii against restraint stress-induced memory dysfunction in mice through modulating intestinal inflammation and permeability—a study based on the gut–brain axis hypothesis. Frontiers in Pharmacology 2021, 12,
- Stenman, L.K.; Patterson, E.; Meunier, J.; Roman, F.J.; Lehtinen, M.J. Strain specific stress-modulating effects of candidate probiotics: A systematic screening in a mouse model of chronic restraint stress. Behav Brain Res 2020, 379, 112376-112376.10.1016/j.bbr.2019.112376
- Tian, T.; Xu, B.; Qin, Y.; Fan, L.; Chen, J.; Zheng, P.; Gong, X.; Wang, H.; Bai, M.; Pu, J., et al. Clostridium butyricum miyairi 588 has preventive effects on chronic social defeat stress-induced depressive-like behaviour and modulates microglial activation in mice. Biochemical and biophysical research communications 2019, 516, 430-436.10.1016/j.bbrc.2019.06.053
- Burokas, A.; Arboleya, S.; Moloney, R.D.; Peterson, V.L.; Murphy, K.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Targeting the microbiota-gut-brain axis: Prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biological psychiatry 2017, 82, 472-487
- Karen, C.; Shyu, D.J.H.; Rajan, K.E. Lactobacillus paracasei supplementation prevents early life stress-induced anxiety and depressive-like behavior in maternal separation model-possible involvement of microbiota-gut-brain axis in differential regulation of microrna124a/132 and glutamate receptors. Frontiers in Neuroscience 2021, 15, 719933.10.3389/fnins.2021.719933
- Sun, X.; Zhang, H.-F.; Ma, C.-L.; Wei, H.; Li, B.-M.; Luo, J. Alleviation of anxiety/depressive-like behaviors and improvement of cognitive functions by lactobacillus plantarum wlpl04 in chronically stressed mice. Can J Infect Dis Med Microbiol 2021, 2021, 6613903-6613903.10.1155/2021/6613903
- Xiao, Q.; Shu, R.; Wu, C.; Tong, Y.; Xiong, Z.; Zhou, J.; Yu, C.; Xie, X.; Fu, Z. Crocin-i alleviates the depression-like behaviors probably via modulating “microbiota-gut-brain” axis in mice exposed to chronic restraint stress. Journal of Affective Disorders 2020, 276, 476-486.https://doi.org/10.1016/j.jad.2020.07.041
- Keightley, P.C.; Koloski, N.A.; Talley, N.J. Pathways in gut-brain communication: Evidence for distinct gut-to-brain and brain-to-gut syndromes. The Australian and New Zealand journal of psychiatry 2015, 49, 207-214.10.1177/0004867415569801
- Brun, P.; Gobbo, S.; Caputi, V.; Spagnol, L.; Schirato, G.; Pasqualin, M.; Levorato, E.; Palù, G.; Giron, M.C.; Castagliuolo, I. Toll like receptor-2 regulates production of glial-derived neurotrophic factors in murine intestinal smooth muscle cells. Molecular and Cellular Neuroscience 2015, 68, 24-35.https://doi.org/10.1016/j.mcn.2015.03.018
- Amini-Khoei, H.; Haghani-Samani, E.; Beigi, M.; Soltani, A.; Mobini, G.R.; Balali-Dehkordi, S.; Haj-Mirzaian, A.; Rafieian-Kopaei, M.; Alizadeh, A.; Hojjati, M.R., et al. On the role of corticosterone in behavioral disorders, microbiota composition alteration and neuroimmune response in adult male mice subjected to maternal separation stress. International Immunopharmacology 2019, 66, 242-250.10.1016/j.intimp.2018.11.037
- Moussaoui, N.; Larauche, M.; Biraud, M.; Molet, J.; Million, M.; Mayer, E.; Taché, Y. Limited nesting stress alters maternal behavior and in vivo intestinal permeability in male wistar pup rats. PLoS One 2016, 11, e0155037-e0155037.10.1371/journal.pone.0155037
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus nerve as modulator of the brain-gut axis in psychiatric and inflammatory disorders. Frontiers in psychiatry 2018, 9, 44-44.10.3389/fpsyt.2018.00044
- Foster, J.A.; Rinaman, L.; Cryan, J.F. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiology of Stress 2017, 7, 124-136.https://doi.org/10.1016/j.ynstr.2017.03.001
- Lam, Y.Y.; Ha, C.W.Y.; Campbell, C.R.; Mitchell, A.J.; Dinudom, A.; Oscarsson, J.; Cook, D.I.; Hunt, N.H.; Caterson, I.D.; Holmes, A.J., et al. Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice. PLoS One 2012, 7, e34233-e34233.10.1371/journal.pone.0034233
- Cani, P.D.; Neyrinck, A.M.; Fava, F.; Knauf, C.; Burcelin, R.G.; Tuohy, K.M.; Gibson, G.R.; Delzenne, N.M. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 2007, 50, 2374-2383.10.1007/s00125-007-0791-0
- Murray, E.; Sharma, R.; Smith, K.B.; Mar, K.D.; Barve, R.; Lukasik, M.; Pirwani, A.F.; Malette-Guyon, E.; Lamba, S.; Thomas, B.J., et al. Probiotic consumption during puberty mitigates lps-induced immune responses and protects against stress-induced depression- and anxiety-like behaviors in adulthood in a sex-specific manner. Brain, Behavior, and Immunity 2019, 81, 198-212.https://doi.org/10.1016/j.bbi.2019.06.016
- Yamagishi, N.; Omata, Y.; Aoki-Yoshida, A.; Moriya, N.; Goto, T.; Toyoda, A.; Aoki, R.; Suzuki, C.; Takayama, Y. Comparison of gut tight junction gene expression in c57bl/6j and balb/c mice after chronic social defeat stress. Japan Agricultural Research Quarterly: JARQ 2019, 53, 41-46.10.6090/jarq.53.41
- Lin, R.; Wang, Z.; Cao, J.; Gao, T.; Dong, Y.; Chen, Y. Role of melatonin in murine "restraint stress"-induced dysfunction of colonic microbiota. Journal of microbiology (Seoul, Korea) 2021, 59, 500-512.10.1007/s12275-021-0305-7
- Rincel, M.; Lépinay, A.L.; Delage, P.; Fioramonti, J.; Théodorou, V.S.; Layé, S.; Darnaudéry, M. Maternal high-fat diet prevents developmental programming by early-life stress. Translational psychiatry 2016, 6, e966-e966.10.1038/tp.2016.235
- Teixeira, D.; Cecconello, A.L.; Partata, W.A.; de Fraga, L.S.; Ribeiro, M.F.M.; Guedes, R.P. The metabolic and neuroinflammatory changes induced by consuming a cafeteria diet are age-dependent. Nutritional Neuroscience 2019, 22, 284-294.10.1080/1028415X.2017.1380892
- Wait, J.; Burns, C.; Jones, T.; Harper, Z.; Allen, E.; Langley-Evans, S.C.; Voigt, J.-P. Early postnatal exposure to a cafeteria diet interferes with recency and spatial memory, but not open field habituation in adolescent rats. Developmental Psychobiology 2021, 63, 572-581.https://doi.org/10.1002/dev.22063
- de Sousa Rodrigues, M.E.; Bekhbat, M.; Houser, M.C.; Chang, J.; Walker, D.I.; Jones, D.P.; Oller do Nascimento, C.M.P.; Barnum, C.J.; Tansey, M.G. Chronic psychological stress and high-fat high-fructose diet disrupt metabolic and inflammatory gene networks in the brain, liver, and gut and promote behavioral deficits in mice. Brain, Behavior, and Immunity 2017, 59, 158-172.https://doi.org/10.1016/j.bbi.2016.08.021
- Rohr, M.W.; Narasimhulu, C.A.; Rudeski-Rohr, T.A.; Parthasarathy, S. Negative effects of a high-fat diet on intestinal permeability: A review. Advances in Nutrition 2020, 11, 77-91.10.1093/advances/nmz061
- Bridgewater, L.C.; Zhang, C.; Wu, Y.; Hu, W.; Zhang, Q.; Wang, J.; Li, S.; Zhao, L. Gender-based differences in host behavior and gut microbiota composition in response to high fat diet and stress in a mouse model. Scientific Reports 2017, 7, 10776.10.1038/s41598-017-11069-4
- del Bas, J.M.; Guirro, M.; Boqué, N.; Cereto, A.; Ras, R.; Crescenti, A.; Caimari, A.; Canela, N.; Arola, L. Alterations in gut microbiota associated with a cafeteria diet and the physiological consequences in the host. International Journal of Obesity 2018, 42, 746-754.10.1038/ijo.2017.284
- Kaakoush, N.O.; Martire, S.I.; Raipuria, M.; Mitchell, H.M.; Nielsen, S.; Westbrook, R.F.; Morris, M.J. Alternating or continuous exposure to cafeteria diet leads to similar shifts in gut microbiota compared to chow diet. Molecular Nutrition & Food Research 2017, 61, 1500815.https://doi.org/10.1002/mnfr.201500815
- Hatton-Jones, K.M.; du Toit, E.F.; Cox, A.J. Effect of chronic restraint stress and western-diet feeding on colonic regulatory gene expression in mice. Neurogastroenterology & Motility 2021, 00, e14300.https://doi.org/10.1111/nmo.14300
- Ranyah Shaker, M.L.; Alfawaz, H.; Almnaizel, A.T.; Hassan, W.M.; Ramesa Shafi, B.; Nadine, M.S.M.; Bjørklund, G.; El-Ansary, A. High-fat diet-induced obesity and impairment of brain neurotransmitter pool. Translational Neuroscience 2020, 11, 147-160.http://dx.doi.org/10.1515/tnsci-2020-0099
- Saiyasit, N.; Chunchai, T.; Prus, D.; Suparan, K.; Pittayapong, P.; Apaijai, N.; Pratchayasakul, W.; Sripetchwandee, J.; Chattipakorn, M.D.P.D.N.; Chattipakorn, S.C. Gut dysbiosis develops before metabolic disturbance and cognitive decline in high-fat diet–induced obese condition. Nutrition 2020, 69, 110576.https://doi.org/10.1016/j.nut.2019.110576
