Intestinal fibrosis is one of the most threatening complications of Crohn’s disease. Endoscopic and surgical approaches are currently the only options available and there is an urgent need for targeted anti-fibrotic therapy. Several molecules investigated in preclinical studies, which are awaiting clinical trials in humans, have proven effective in CD stricturing phenotype and may be available in the near future as additional weapons in preventing or reversing intestinal fibrosis.
- antifibrotic therapy
- Crohn’s disease
- IBD
- intestinal fibrosis
1. Introduction
2. Overview of the Main Mechanisms of Intestinal Fibrosis in CD
As mentioned above, the abnormal inflammatory stimulus due to CD is associated with uncontrolled activation of mesenchymal cells, resulting in excessive ECM deposition [10]. In addition, an imbalance between matrix metalloproteinases (MMPs) and their inhibitors, tissue inhibitors of metalloproteinases (TIMPs), appears to be associated with increased ECM deposition and subsequent tissue fibrosis [11]. These mechanisms, together with the thickening of the muscle layer due to hyperplasia and hypertrophy of the smooth muscle cells [12], determine the development of fibrostenotic strictures in CD.
Here we will briefly outline the main players underlying the process of intestinal fibrosis. The correlation between these factors is in most cases unknown and elusive. The figure below shows some of these cellular and molecular players and their interaction in the fibrogenic process leading to intestinal stricture formation.
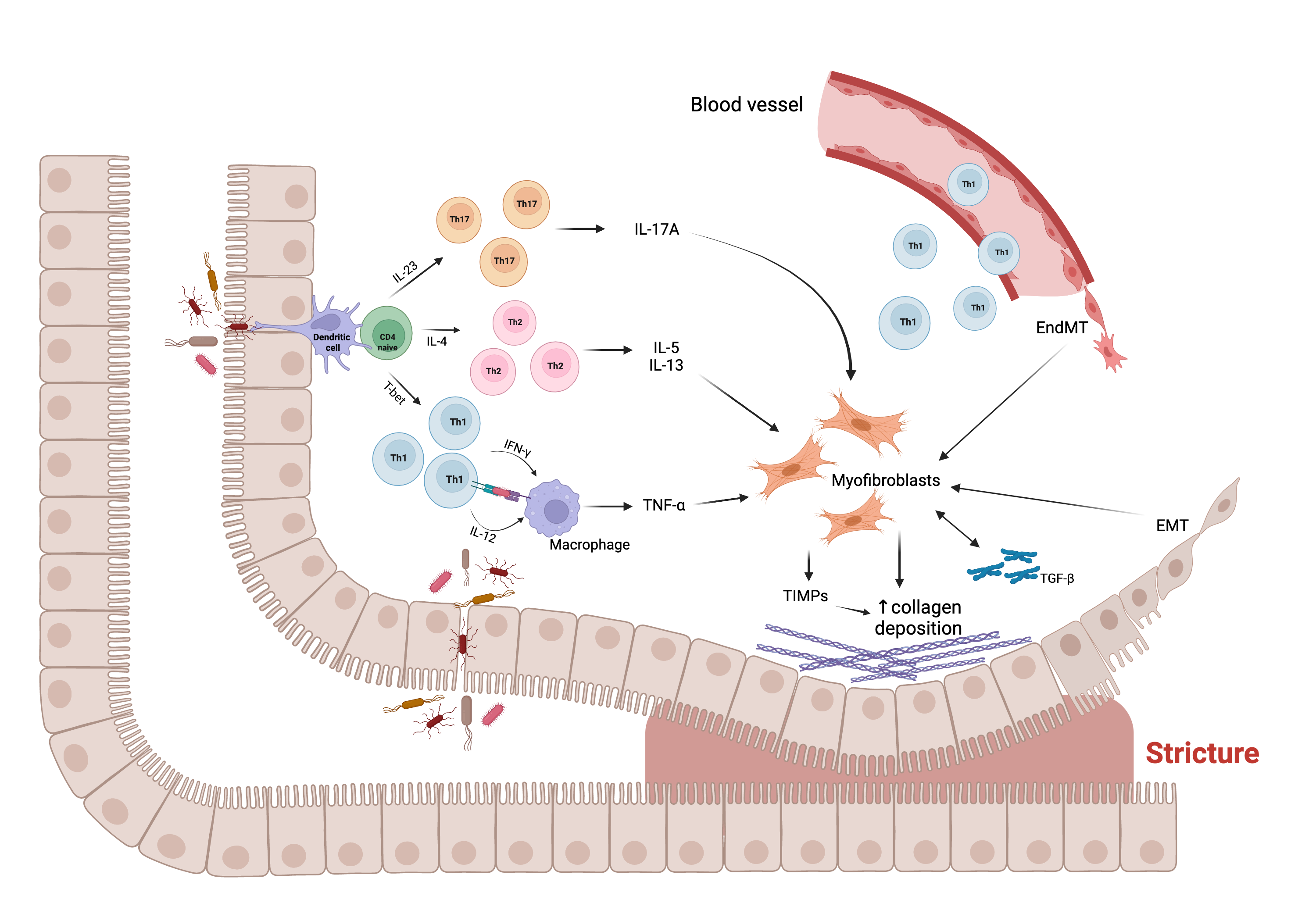
Figure. Main molecular and cellular mechanisms, and their interaction, underlying the fibrogenic process leading to stricture formation in Crohn’s disease (CD). Intestinal mucosal infiltration of CD4+ T cells represents a key characteristic of CD. Multiple Th subsets have been identified, with different role in the fibrogenic process. The cross-talk between macrophage and T cells, sustained by Th1 pro-inflammatory cytokines, including interferon (IFN)-ɣ and interleukin (IL)-12, results in the production of tumor necrosis factor (TNF)-α, which promotes myofibroblast production of transforming growth factor (TGF)-β1. The latter inhibits the production of matrix metalloproteinases (MMPs) and favors the production of tissue inhibitor of metalloproteinases (TIMPs), which causes abnormal collagen deposition, with consequent fibrosis and stricture formation. In addition, Th2 and Th17 cells have a pro-fibrotic role through the production of pro- fibrotic cytokines, especially IL-17A, which induces intestinal myofibroblast secretion of collagen and TIMPs and significantly inhibits myofibroblast migration. The fibrotic process is also sustained by epithelial-mesenchymal transition (EMT) and endothelial-mesenchymal transition (EndMT), constantly evolving processes in which epithelial and endothelial cells acquire fibroblast characteristics. Abbreviations: EMT, epithelial-mesenchymal transition; EndMT, endothelial- mesenchymal transition; IL, interleukin; IFN, interferon; T-bet, T-box transcription factor; TGF, transforming growth factor; Th, T helper cell; TIMP, tissue inhibitor of metalloproteinase; TNF, tumor necrosis factor; ↑, increase. Created with “BioRender.com”, 21 Jan 2022.
2.1. Main Cells Involved in Fibrogenesis
The main cells involved in the fibrogenesis process are mesenchymal cells, which are specifically committed to the production of collagen. The main players in this process are fibroblasts, myofibroblasts, and smooth muscle cells [13]. In particular, the inflammatory stimulus associated with CD seems to determine the activation of tissue fibroblasts and the migration of non-resident fibroblasts at the site of damage. These fibroblasts, under the stimulus of growth factors such as transforming growth factor (TGF)-β, may differentiate into myofibroblasts, capable of producing ECM. Similarly, smooth muscle cells are able to differentiate into myofibroblasts and likewise myofibroblasts can differentiate into smooth muscle cells and lead to the thickening of the muscularis propria and the formation of strictures [14]. Finally, the possible role of inflammation-induced differentiation of epithelial and endothelial cells into ECM-secreting mesenchymal cells should be considered, according to the mechanisms of epithelial-mesenchymal transition (EMT) and endothelial-mesenchymal transition (EndMT) [15,16]. EMT is a constantly evolving process in which epithelial cells acquire a migratory function and develop fibroblast characteristics. Similarly, EndMT is a process in which endothelial cells acquire fibroblast characteristics.
2.2. Molecular Mediators of Fibrosis
It is assumed that several cytokines can actively participate into the fibrogenesis process. Among these, the role of TGF-β is certainly predominant [17]. More specifically, the TGF-β1 isoform promotes collagen synthesis and fibroblast contraction in the mucosa of patients with fibrostenosing CD, acting through the Smad2-Smad3 molecular pathway and the regulation of TIMPs. Other cytokines related to organ fibrosis and with an emerging role in intestinal fibrosis, besides their known pro-inflammatory properties, are those belonging to the interleukin (IL)-1 family, including IL-1, IL-33, and IL-36 [18–21]. CD4+ T cells play a crucial role in the pathogenesis of CD and several T helper (Th) subsets have been identified, with different roles. While T-regulatory cells prevail in normal conditions, the Th1 subset appears predominantly pro-inflammatory, whereas Th2 and Th17 subsets appear to have both pro-inflammatory and pro-fibrogenic roles [22,23]. In particular, Th17 cells produce both IL-17 and IL-22 with a possible contrasting effect on intestinal fibrogenesis [24]. The role of cytokines belonging to the IL-17 family is well established, especially that of the IL-17A, as it induces intestinal myofibroblast secretion of collagen and TIMPs and significantly inhibits myofibroblast migration [25]. A possible role in this process has also been ascribed to the IL-17E (also known as IL-25), whose production in the human gut is reduced by tumor necrosis factor (TNF)-α and enhanced by TGF-β1 [26]. However, the pro-fibrotic role of IL-17E in CD has been questioned by the finding of no-significant difference on IL-17E levels in strictured compared to non- strictured CD tissues [25]. Fibroblast activation protein (FAP) is another protein typically produced by activated fibroblasts during wound healing and implicated in the fibrotic evolution of tissue damage [27]. FAP has been shown to be highly overexpressed in the submucosa and the muscle layer of stenotic CD, compared to non-stenotic CD [28]. In addition, other growth factors have an established role in gut fibrosis, especially the basic fibroblast growth factor (bFGF), which is overexpressed in patients with stricturing CD phenotype [29]. Concerning the role of TNF-α family members, there is growing evidence about the TNF-like cytokine 1A (TL1A), secreted from immune cells and binding the death domain receptor 3 (DR3) expressed on intestinal myofibroblasts [30]. TL1A is highly expressed in the fibrotic tissue of CD patients and a gene variant of the TL1A gene is associated with a higher risk of fibrotic strictures [31]. Finally, a possible role of neutrophil extracellular traps (NETs) has recently emerged. NETs are large, extracellular, web-like structures extruded by neutrophils under various conditions, especially immune response towards pathogens, representing a defense mechanism that, if dysregulated, can contribute to the pathogenesis of immune-related disorders [32]. NETs have been shown to mediate the in vitro activation of fibroblasts into myofibroblasts in fibrotic interstitial lung disease [33], and it has been suggested that this role may also be played in the gut [34].
2.3. MicroRNAs
MicroRNAs (miRNAs) are small non-coding ribonucleic acid (RNA) sequences that interfere with mRNA, causing, in most cases, an inhibition of translation [35]. The role of miRNAs on intestinal fibrosis in CD is relatively poorly established. Two families of miRNAs, miRNA-29 and miRNA-200, appear to be involved in this process. Specifically, miRNA-29a, -29b, and -29c were found to be down-regulated in CD strictured mucosa, with a role for miRNA-29b in modulating in vitro the expression of collagen I and III [36]. The miRNA 200 family appears to play a protective role against the development of EMT [37,38].
2.4. The Role of Gut Microbiota
The human gut hosts a complex and abundant aggregation of microbes, collectively referred to as the gut microbiota, whose compositional and metabolic alterations, defined as dysbiosis, have a pivotal role in IBD pathogenesis [39]. All intestinal immune and non- immune cell types express the pathogen recognition receptors, such us Toll-like receptors (TLRs) and nucleotide-binding and oligomerization domain (NOD)-like receptors, which provide the ability to respond to pathogen-associated molecular patterns [40]. It has been reported that in primary human intestinal fibroblasts, the TLR5 ligand flagellin (present in all flagellated bacteria) induces a pro-inflammatory and pro-fibrotic phenotype [41]. More recently, it was confirmed that flagellin derived from adherent-invasive Escherichia coli (AIEC), a micro-organism frequently isolated in the ileal tissue of CD, could bind the TLR5 expressed in intestinal epithelium, determining the expression of the IL-33 receptor (ST2), which is crucial for the development of intestinal fibrosis [42]. Supporting the hypothesis of a causal role of microbiota on intestinal fibrogenesis, the recurrence of NOD2 variants in CD patients with fibrostenotic phenotype has been highlighted [43]. Finally, several studies on animal models of intestinal fibrosis have shown a pro-fibrotic activity of the gut microbiota [44,45].
2.5. Matrix Stiffness
Another novel mechanism associated with intestinal fibrosis in CD patients is matrix stiffness. Resistance to matrix deformation has been shown to be an important mediator of cellular behavior [46]. Cell proliferation and differentiation are assumed to increase with matrix stiffness. This is true even for human colonic fibroblasts, which have been proved to be activated to a profibrotic phenotype by matrix stiffness [47,48]. An increase in ECM stiffness would be associated with a morphological alteration of fibroblasts, actin stress fiber formation and focal adhesion, promoting fibroblast proliferation and activation, even in the absence of inflammatory stimulation. This finding suggests that intestinal fibrosis would have a self-propagation mechanism independent of the inflammatory stimulus and matrix stiffness may have a role in this process.
2.6.“Creeping Fat”
Mesenteric fat and its hypertrophy, known as ‘creeping fat’, have an emerging role in the pathogenesis of CD fibrostenotic phenotype [49]. The role of creeping fat in intestinal fibrogenesis is associated with the production of adipokines that promote a shift of the macrophage compartment towards M2 macrophage dominance, resulting in increased production of TGF-β1 [50]. Furthermore, creeping fat has been shown to be associated with smooth muscle hypertrophy [51].
3. Therapeutic Approaches of Crohn’s Disease
To date, there are no medical therapies available to prevent or reverse intestinal fibrosis in CD. In patients with intestinal obstruction due to CD with a fibrostenosing phenotype, initial treatment consists of nasogastric decompression, bowel rest, intravenous hydration, and electrolyte replacement. Subsequent management is dependent on strictures’ inflammation degree and morphometrics, such as location and length, assessed by biomarkers (e.g., C-reactive protein, erythrocyte sedimentation rate, and fecal calprotectin), endoscopy, computed tomography, or magnetic resonance imaging. Current complications (such as phlegmon, abscess, dysplasia, or malignancy) and patient preferences should also be taken into account [5,54].
3.1. Current Medical Options
3.2. Endoscopic and Surgical Management
3.3. Promising Anti-Fibrotic Therapy in CD
Increasing knowledge of the molecular mechanisms underlying intestinal fibrosis has enabled the identification of anti-fibrotic therapeutic targets. At present, although there is no therapy capable of treating or reversing intestinal fibrosis in CD, several pre- clinical studies have been conducted in vivo, ex vivo, and in vitro, with encouraging results. Herein, we report the most promising anti-fibrotic therapeutic targets known to date and the relevant target-specific molecules under investigation (Table below).
3.3.1. Targeting TGF-β Pathways
The most promising target for anti-fibrotic therapy is TGF-β, the principal molecular mediator of fibrogenesis, and its signaling pathways.
-
Several studies on fibrosis of other tissues have shown that TGF-β1 production was strongly stimulated by the local activation of angiotensin II [72,73,74], the main effector of the renin-angiotensin system, whose activity is increased in the colonic mucosa of CD patients [75]. For this reason, it was assumed that angiotensin conversing enzyme (ACE) inhibitors and sartans (angiotensin II receptor antagonists), which typically act as anti-hypertensives, could also play a role in the process of intestinal fibrogenesis. The first ACE-inhibitor investigated was captopril, which showed to be effective in preventing colonic fibrosis in 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis in rats. Its anti-fibrotic action has been assumed to derive from blocking TGF-β1 overexpression and/or from a direct down-regulation of TGF-β1 transcripts [76]. Moreover, transanal administration of enalaprilat has been shown to be effective in preventing colonic fibrosis in a dextran sulfate sodium (DSS)-induced colitis model [77]. More recently, losartan, an antagonist of the angiotensin II receptor, was investigated and exhibited a pleiotropic effect, reducing TGF-β1 concentration and significantly improving the macro- and microscopic scores of fibrosis in the colonic wall of rats [78];
-
Based on the known antagonistic relationship between the TGF-β/Smad pathway and the peroxisome proliferator-activated receptor (PPAR)γ, a member of ligand-activated transcription factors of nuclear hormone receptor superfamily [79,80], the effect of a novel 5-ASA analog (named GED-0507-34 Levo), able to activate PPARγ, has been investigated. GED-0507-34 Levo showed improvement of intestinal fibrosis in DSS-induced chronic colitis in mice, reducing the activation of myofibroblasts and the expression of the main pro-fibrotic molecules including TGF-β, Smad3, IL-13 and connective tissue growth factor (CTGF) [81]. Similarly, it has been shown that other PPARγ agonists, usually employed in the treatment of diabetes, such as troglitazone and rosiglitazone, may be useful in counteracting the fibrogenic process by suppressing TGF-β1-induced synthesis of collagen, fibronectin, and α-smooth muscle actin in human primary intestinal myofibroblasts [82];
-
Another target signaling pathway induced by TGF-β1 but also by matrix stiffness is that of Rho/Rho chinase (ROCK) [83]. The first ROCK inhibitors studied were CCG-1423, CCG-100602, and CCG-203971, which, by inhibiting RhoA signaling in myofibroblasts, induced a significant anti-fibrotic activity [84,85]. These molecules, however, showed an unacceptable toxicity profile, especially with regard to cardiovascular side effects [86]. For this reason, the effect of a locally acting ROCK inhibitor (AMA0825) was investigated. This molecule prevented and reversed intestinal fibrosis in vitro and ex vivo by diminishing TGF-β1-induced activation of myocardine-related transcription factor and p38 mitogen-activated protein kinase (MAPK) and increasing autophagy in fibroblasts, with a good tolerability profile [87]. Combining AMA0825 with anti-inflammatory agents (such as anti-TNF-α) in vivo ameliorated inflammation but also prevented accumulation of fibrotic tissue, underscoring the importance of combination therapy;
-
Other compounds have been shown to downregulate the TGF-β signaling. These include cilengitide, which is an Arg-Gly-Asp (RGD)-containing αVβ3 integrin inhibitor, that is able to decrease TGF-β1 activation and development of fibrosis in chronic TNBS-induced colitis [88]. More recently, anti-fibrotic intestinal efficacy has been proposed for two molecules approved for the treatment of idiopathic pulmonary fibrosis, namely pirfenidone and nintedanib [89,90]. In particular, pirfenidone, an orally delivered pyridine derivative that suppresses TGF-β and TNF-α signals, inhibited, both in vivo and in vitro, intestinal fibroblast proliferation and motility and reduced collagen production through different TGF-β1 signaling pathways, including those of suppressor of mothers against decapentaplegic (Smad), phosphatidylinositol-3-kinase (PI3K)/AKT, MAPK, and mechanistic target of rapamycin (mTOR) [91,92,93,94]. Therefore, this molecule is of great interest and has important therapeutic potential, but needs further studies to better clarify its mechanism of action, efficacy, and safety [95]. No studies are yet available on the usefulness in intestinal fibrosis of nintedanib, a small oral molecule inhibitor of tyrosine kinase receptors, such as platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), and vascular endothelial growth factor (VEGF) receptors. Finally, an anti-fibrotic action of maggot extract was described by downregulating the TGF-β1/Smad pathway via upregulation of nuclear factor erythroid 2-related factor 2 (Nrf2) expression [96].
3.3.2. Targeting TIMP/MMP Balance
3.3.3. Targeting VEGF
3.3.4. Targeting FAP
3.3.5. Targeting EMT
3.3.6. Targeting the Endogenous Cannabinoid System
3.3.7. Targeting IL-17
3.3.8. Targeting IL-36
3.3.9. Targeting TL1A
3.3.10. Targeting Both TNF-α and IL-17
3.3.11. Targeting AXL Pathway
3.3.12. Targeting NETs
3.3.13. Targeting miRNAs
3.3.14. Targeting Matrix Stiffness
3.3.15. Targeting Intestinal Microbiota
|
TARGET |
AGENT |
MECHANISM |
MODEL |
REFERENCE |
|
TGF-b pathways |
Captopril |
↓ TGF-b1 expression and/or TGF-b1 transcript |
TNBS-colitis |
78 |
|
Transanal enalaprilat |
↓ TGF-b signaling pathway |
DSS-colitis |
79 |
|
|
Losartan |
↓ TGF-b1 expression |
TNBS-colitis |
80 |
|
|
GED-0507-34 Levo |
PPAR-γ activation |
DSS-colitis |
83 |
|
|
Troglitazone, Rosiglitazone |
PPAR-γ activation |
HIFs |
84 |
|
|
CCG-1423, CCG-100602, CCG-203971 |
ROCK inhibition |
CCD18-co HIFs |
87 |
|
|
AMA0825 |
ROCK inhibition |
DSS- and T-cell transfer-colitis, HIFs |
89 |
|
|
Cilengitide |
αVβ3 integrin inhibition |
TNBS-colitis |
90 |
|
|
Pirfenidone |
Smad, PI3K/AKT, MAPK, and mTOR signaling pathways inhibition |
HIFs, DSS-colitis, RIF |
93-96 |
|
|
Maggot extract |
↑ Nrf2 expression |
DSS-colitis |
98 |
|
|
TIMP/MMP balance |
Thalidomide |
Altered TIMP/MMPs balance and ECM degradation |
TNBS-colitis |
100 |
|
VEGF |
Bevacizumab |
↓ collagen deposition |
n.a. |
n.a. |
|
FAP |
Anti-FAP Ab |
FAP inhibition |
HIFs |
104 |
|
EMT |
rhBMP-7 |
EMT inhibition |
TNBS-colitis |
111 |
|
miRNA200b-containing microvescicles |
EMT inhibition |
TNBS-colitis, IEC-6 |
112 |
|
|
Endogenous cannabinoid system |
MAEA |
↓ collagen production and ↑ myofibroblasts migration |
Human organ culture biopsies, LPMCs, and HIFs |
117 |
|
IL-17 |
Anti-IL17 Ab |
↓ profibrogenic cytokines and MMP/TIMPs balance alteration |
TNBS-colitis |
119 |
|
IL-36 |
Anti-IL36R Ab |
↓ collagen production, MMPs, IL6 signaling, and EMT |
DSS- and TNBS-colitis |
124 |
|
TL1A |
Anti-TL1A Ab |
TGF-1/Smad3 signaling pathway inhibition |
T-cell transfer-colitis |
125 |
|
TNF-α and IL-17 |
ABT-122 |
n.a. |
n.a. |
n.a. |
|
AXL pathway |
BGB324 |
↓ matrix stiffness and TGF-b1-induced fibrogenesis |
CCD-18co, TNBS-colitis |
131 |
|
NETs |
PAD4 inhibitors |
↓ NETs-derived fibrosis |
n.a. |
n.a. |
|
miRNA |
miRNA29 |
↓ TGF-β1-induced collagen expression |
Human fibroblasts cultures |
36 |
|
miRNA200 |
↓ ZEB1 and ZEB2, EMT inhibition |
Intestinal epithelial cells |
38 |
|
|
Matrix Stiffness |
b-aminopropionitrile |
↑ MMP3 activity and ↓ ECM contraction |
HIFs |
137 |
|
Gut microbiota |
Probiotics and prebiotics |
Modulation fibrotic pathways |
Mouse and cellular models |
138-142 |
Abbreviations: Ab, antibody; CCD-18Co, noncancerous colon fibroblast; DSS, dextran sulfate sodium; ECM, extracellular matrix; EMT, epithelial-mesenchymal transition; EndMT, endothelial mesenchymal transition; FAP, fibroblast activation protein; HIF, human intestinal fibroblast; IEC, intestinal epithelial cell; IL, interleukin; LPMC, lamina propria mononuclear cell; MAEA, methanandamide; MAPK, mitogen-activated protein kinase; mTOR, mechanistic target of rapamycin; miRNA, micro ribonucleic acid; MMP, matrix metalloproteinase; n.a., not available; NET, neutrophil extracellular trap; Nrf2, nuclear factor erythroid 2-related factor 2; PAD4, peptidylarginine deiminase 4; PI3K, phosphatidylinositol-3-Kinase; PPAR, peroxisome proliferator-activated receptor; rhBMP-7, recombinant human bone morphogenic protein-7; RIF, radiation-induced intestinal fibrosis; ROCK, Rho/Rho chinase; Smad, suppressor of mothers against decapentaplegic; TGF, transforming growth factor; TIMP, tissue inhibitor of metalloproteinase; TL1A, TNF-like cytokine 1A; TNBS, 2,4,6-trinitrobenzene sulfonic acid; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor; ZEB, zinc finger E-box binding homeobox; ↑, increase; ↓, decrease.
4. Major Challenge for Anti-Fibrotic Agents Development
There are numerous pitfalls in identifying anti-fibrotic drugs for CD. First, experimental fibrosis in cellular and animal models does not necessarily resemble human fibrosis [139]. Cells may behave differently in vitro and in vivo and single cell studies often do not reproduce the complex in vivo cellular network. For this reason, 3D models are under development to reproduce the natural microenvironment as closely as possible to that in vivo [140,141]. Moreover, the targeted molecules often represent a small component of the complex molecular maze underlying the fibrotic process. The target molecules may even have multiple functions on the intestinal tissue, with the risk of targeting processes implicated in physiological tissue remodeling, resulting in negative effects. In addition, to date there are neither fibrosis biomarkers, nor diagnostic tools that can be used to identify and quantify the overall fibrotic burden in CD patients, especially in the early stages, when anti-fibrotic therapy may be mostly effective [142,143]. Finally, there is an urgent need of end points that can be used to assess the efficacy of anti-fibrotic agents in clinical trials. For this reason, several groups of renowned IBD experts have reached expert consensus on this matter [6,144]. In particular, a core set of 13 end-points (i.e., complete clinical response, long-term efficacy, sustained clinical benefit, treatment failure, radiological remission, normal quality of life, clinical remission without steroids, therapeutic failure, deep remission, complete absence of occlusive symptoms, symptom- free survival, bowel damage progression, and no disability) were considered critical [144]. The combination of improved clinical, endoscopic and/or radiological features seems appropriate to define a successful treatment [6]. The need for intervention within 24-48 weeks from medical therapy has been proposed as the most accurate end-point to assess anti-fibrotic agents in pharmacological trials.
Despite the urgency for anti-fibrotic therapy and the numerous molecules identified as potential anti-fibrotics in CD, no phase III clinical trial is currently ongoing or recruiting to our knowledge (according to ClinicalTrials.gov, as of 19 January 2022).
This entry is adapted from the peer-reviewed paper 10.3390/cells11030429
