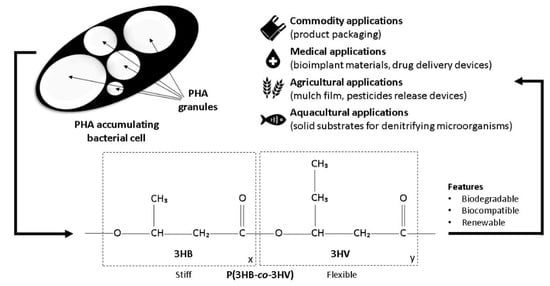
1. P(3HB-co-3HV) Properties and Applications
Poly(3-hydroxybutyrate) (P(3HB)) is a relatively stiff and brittle polyester with poor elongation at break [
15]. It is a fragile material, and its mechanical properties deteriorate with time due to secondary crystallization accompanied by aging at room temperature, which is the major cause of its brittleness [
16]. Although the lack of elasticity causes a drawback in its application as packaging materials, its high mechanical properties are applicable as bone tissues aid in supporting body weight. P(3HB) facilitates reconstructive osteogenesis. P(3HB) and its biocomposite incorporated with 20 wt% hydroxyapatite, which makes up 65–70% of the bone matrix, show pronounced osteoplastic properties owing to their slow degradation that corresponds to the growth of new bones. Powdered P(3HB) and P(3HB)/tienam are excellent antibacterial bone filling materials that contribute to 1-fold lower growth and complete growth inhibition of
Staphylococcus aureus post surgery, respectively [
17].
The incorporation of the C
5 3-hydroxyvalerate (3HV) monomer into P(3HB) results in P(3HB-
co-3HV) with decreased crystallinity, thus leading to decreased stiffness, decreased brittleness, and enhanced biodegradability compared to that of P(3HB) [
18]. The properties of P(3HB-
co-3HV) are dependent on the ratio of the two monomers where the 3HB monomer contributes stiffness, and the 3HV monomer contributes flexibility to the copolymer. The composition of the 3HV monomer determines the defection of the P(3HB) lamellae crystals, leading to the disruption of its crystallinity and resulting in improved polymer flexibility (
Figure 1) [
19]. The lower degree of crystallinity and melting point of P(3HB-
co-3HV) lead to a higher degradation rate that is directly proportional to the molar fraction of 3HV of the copolymer compared to that of P(3HB) [
18]. The 3HV fraction contributes to a greater amorphous region for enzymatic attacks that leads to enhanced and adjustable biodegradability for applications such as implants for bone support, stents for artery support in angioplasty, and drug delivery carriers. Although P(3HB-
co-3HV) has a 2-fold lower maximum water permeability than poly(lactic acid) which is another biodegradable aliphatic polyester of great biotechnological importance, causing lower hydrolytic degradation due to lower water uptake, the degradation rate of P(3HB-
co-3HV)-based biomedical devices are adjustable with molar fraction of 3HV [
18,
20,
21]. Hydrophilic poly(ethylene glycol) and monomethoxy poly(ethylene glycol) can also be incorporated into P(3HB-
co-3HV) to form nanoparticles with a hydrophilic outer layer and a hydrophobic inner layer for improved chemical functionalization and compatibility with therapeutic drugs besides benefiting drug release control [
22,
23,
24]. Incorporation of other desired properties for biomedical applications can also be achieved.
Figure 1. Microbial PHA granule, P(3HB-co-3HV) structure, and applications.
P(3HB-
co-3HV) is a potential substitute for petroleum-based plastic packaging material as it possesses high water and aroma (limonene and linalool) barrier properties while having comparable thermal and mechanical properties to that of polypropylene (PP) and low-density polyethylene (LDPE) [
15]. As PP and LDPE are applied extensively for packaging and consumables, which are highly disposable, the substitution with P(3HB-
co-3HV) can contribute to reduced stable solid waste creation of petroleum-based plastics [
47,
48]. Unlike the augmented cytotoxicity by higher 3HV molar fraction, lower 3HV molar fraction causes high stereoregularity, slow crystallization rate, formation of large size spherulites, and secondary crystallization that are discouraging for packaging purposes [
24,
47,
48]. Poly(butylene succinate), poly(butylene adipate-
co-terephthalate), natural rubber, or other polymers with plasticizer or toughness properties can be incorporated to overcome the limitations and extend its application as packaging materials.
Moreover, PHA-based mulch films are potential substitutes for conventional plastic mulch films. Mulching increases crops productivity, increases horticulture products, prevents water evaporation from the soil, prevents soil erosion, reduces water consumption, and controls weeds [
49]. PHA-based mulch films overcome the environmental problems caused by the post-consumption of plastic mulch films made from LDPE, linear low-density polyethylene (LLDPE), and high-density polyethylene (HDPE) due to their poor degradability [
50]. Moreover, the physicochemical properties of P(3HB-
co-3HV) enable the controlled release of herbicides and insecticides. Herbicides and insecticides can be integrated into P(3HB-
co-3HV)-containing pellets and sown along the plantation to be released upon degradation from the pellets depending on the level of pest activity [
51,
52].
On the other hand, endogenous P(3HB-
co-3HV) acts as the electron donor for the denitrification of wastewater in the aquaculture industry. Biomass with PHA-accumulating ability, generally P(3HB) and poly(3-hydroxyvalerate) (P(3HV)), from activated sludge, is employed to remove resulting ammonia from fish excretion and dead animal bodies in circulating water. Unlike the conventional techniques that involve the addition of acetate and ethanol to promote microbial activity, the biomass is precultured for PHA accumulation. The endogenous PHA is used for denitrification that accurately couples with slow metabolic activity in the absence of exogenous carbon source and in the presence of nitrogen [
53,
54]. The exclusion of volatile fatty acids feeding during the denitrification process prevents the contamination with the dissolved organic carbon that lowers the effluent water quality, and the employment of endogenous PHA is more cost-effective compared to feeding extracted PHA to denitrifying bacteria [
55].
2. Bioconversion of Alkyl Alcohols and Organic Acids into P(3HB-co-3HV)
The most common way to incorporate 3HV monomers is by employing a precursor carbon source as a co-substrate along with the main carbon source that contributes to the 3-hydroxybutyrate (3HB) monomer. Precursor carbon sources such as organic acids, alcohols, or some amino acids were studied thoroughly to clarify the metabolic pathways involved and to search for promising precursors of greater potential. Organic acids, especially propionic acid, valeric acid, and their respective salts, are the standard 3HV precursors owing to their wide acceptance among PHA-producing bacteria. However, organic acids can only be added in low concentrations due to their high toxicity to the bacteria, and their high substrate cost causes lower profitability. Although levulinic acid is way more cost-effective than propionic acid and valeric acid, it seems to be a privilege for Cupriavidus necator, and the production mechanism is yet to be clarified [1][2]. Although some amino acids such as threonine, valine, and isoleucine could be employed as 3HV precursors, metabolic engineering of the amino acid biosynthetic pathways is required to convert amino acids into propionyl-CoA, which is essential for 3HV formation. The rare occurrence of alcohols-utilizing ability among PHA-producing bacteria hinders the employment of alkyl alcohols as 3HV precursors despite their potential as cost-effective substitutes for organic acids [3]. In addition to the merit in lowering the substrate cost, naturally occurring carbon sources such as glucose and glycerol or organic wastes can be converted by microorganisms into alkyl alcohols, thus are promising as cost-effective and sustainable bioresources for P(3HB-co-3HV) production [4].
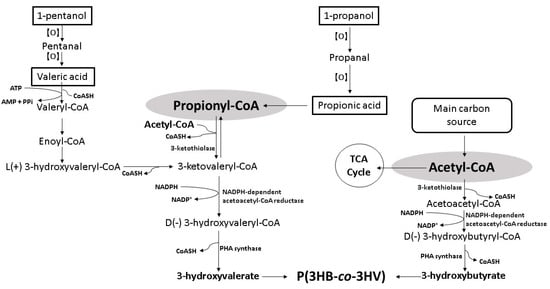
The conversion of organic acid into 3HV starts with
β-oxidation, where propionic acid (C3) is converted into propionyl-CoA, whereas valeric acid (C5) is converted into propionyl-CoA and acetyl-CoA, respectively [
56]. The 3HV monomer is formed from the resulting propionyl-CoA couples with acetyl-CoA and is polymerized to P(3HB-
co-3HV) copolymer with the 3HB monomer. The 3HB monomer is formed from the resulting acetyl-CoA provided majorly by the main carbon source such as oils or sugars (
Figure 2) [
14,
57,
58,
59,
60].
Figure 2. Schematic bioconversion pathway of organic acids and alkyl alcohols into 3HV [
14,
57,
58,
59,
60].
The employment of alkyl alcohols as 3HV precursors is limited to odd carbon number primary alcohols. Primary alcohols are oxidized to aldehydes that can be further oxidized more easily to their respective carboxylic acids. The oxidation processes can occur chemically with the presence of oxidizing agents or biologically with the presence of alcohol dehydrogenase and aldehyde dehydrogenase [
61]. Oxidation of secondary alcohols liberates ketones with no further oxidation due to the oxidatively stable nature of ketones [
62,
63]. Odd carbon number primary alcohols such as 1-propanol or 1-pentanol are oxidized to 1-propanal and 1-pentanal that further oxidized to propanoic acid and valeric acid, respectively. The resulting propionic acid or valeric acid enters
β-oxidation to liberate propionyl-CoA for 3HV formation (
Figure 2) [
14,
57,
58,
59,
60].
Although levulinic acid is a cost-effective 3HV precursor, the catabolic pathway involved is undetermined. Generally, levulinic acid catabolism releases intermediates that are converted via
β-oxidation to release acetyl-CoA and propionyl-CoA for P(3HB-
co-3HV) biosynthesis [
64]. Bacteria capable of using levulinic acid as the 3HV precursor are rare and are mainly
C. necator, with the exception of
Burkholderis sp. IS-01 and
Hydrogenophaga pseudoflava DSM 1034 [
10,
11,
65,
66,
67,
68].
C. necator KHB-8862 and
H. pseudoflava DSM 1034 are two promising strains reported with a high 3HV yield of 0.50 and 1.00 g/g, respectively. However, other studies reported low PHA content and 3HV yield.
3. Techno-Economic and Sustainability Assessment
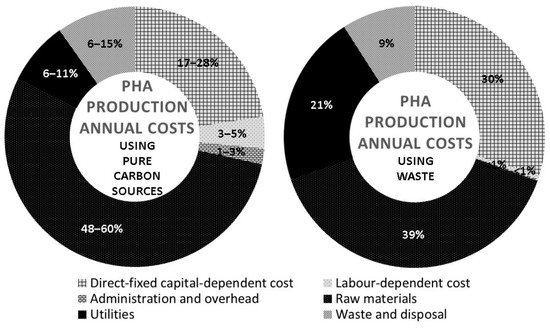
The annual operating costs in PHA production generally include the direct fixed capital-dependent items, labor-dependent items, administration, and overhead expenses, raw materials, utilities, and downstream processing such as waste management. According to the techno-economic analysis conducted by Choi and Lee (1999) for various pure carbon sources, the substrate cost accounted for 48%–60% of the total costs (
Figure 3) [
93]. After excluding the trace elements, which are essentials, pure carbon sources that possess high nutritional value such as glucose, glycerol, starch, methane, oils, and volatile fatty acids are commercial products, and their employment leads to higher substrate cost compared to that of industrial or domestic wastes. Due to higher economic advantage and increasing emphasis on sustainability, the employment of wastes as carbon sources is widely attempted. Theoretically, substituting pure substrates with wastes contributes to a huge reduction in raw material expenses. However, pretreatments are needed for certain wastes to remove impurities and toxins or to adjust pH [
94]. Pretreatments impose additional costs whereby extra chemicals or equipment are necessary with possible individual optimization. Bhattacharyya and co-workers (2015) reported decreased raw material cost to 39% with the employment of wheat stillage, but the utilities cost increased to 21% as compared to that reported by Choi and Lee (1999) (
Figure 3) [
93,
95].
Figure 3. Techno-economic analysis on PHA production annual costs using pure carbon sources and wastes [
93,
95].
As opposed to main carbon sources, where numerous studies have been conducted on various wastes, employing wastes as 3HV precursors is not practical due to the composition inconsistency [
95]. Due to the necessity of propionyl-CoA for 3HV formation, sole reliance on wastes results in the narrow choice to those with propionate or valerate related components; thus, in most cases, a 3HV precursor is still required to achieve sufficient 3HV fraction for the copolymer to be practically useful [
64,
95]. This leads to increased raw material cost as propionic acid and valeric acid, which are widely preferred by PHA-producing bacteria, are high-cost precursors. The potential of 1-propanol and 1-pentanol as alternatives for propionic acid and valeric acid is well-known but lack practicality due to its high toxicity to the majority of bacteria. Since 1996, several PHA-producing bacteria from different genera have been reported to use 1-propanol or/and 1-pentanol as 3HV precursors. The emergence of these bacteria bypasses the bottleneck of precursor dominance by organic acids and enables further innovation in fermentation strategies to develop economically feasible and sustainable production processes. Furthermore, 1-propanol and 1-pentanol are manufactured through well-established oxo synthesis and can be biosynthesized by bacteria from sustainable carbon sources such as glucose, glycerol, and organic wastes, which are abundant in nature.