Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Engineering, Biomedical
|
Oncology
Microfluidic devices are useful tools in studying biological phenomena involving fluid flow, thin tissue culture, and cell mobility; each of these aspects make microfluidic techniques attractive for studying human breast cancer, which is the most commonly occurring cancer in women and the second most common cancer overall worldwide.
- : breast cancer
- microfluidics
- metastasis
1. Introduction
Cell cultures have been major tools used in cellular and molecular biology for generations, and although extremely useful for delineating cellular mechanisms, are poor predictors of physiological responses [1]. The lack of a proper micro-environment with critical communications between different cell types has been the major reason for this failure [2]. Cells reside in highly specialized extracellular matrices (ECMs) that provide mechanical support, determine mechanical properties, and importantly, impart extracellular signals to the cells. The ECM is the noncellular component present within all tissues and organs, providing not only the essential physical scaffolding for the cellular constituents, but also initiates crucial biochemical and biomechanical cues required for tissue morphogenesis and homeostasis [3]. Cell cultures also suffer from the absence of interstitial flow, which plays an important role in the morphogenesis, function, and pathogenesis of tissues. About 20% of the body’s mass is estimated to be made up of interstitial fluid, and much of this fluid is in constant slow motion. In living tissues, interstitial flow is linked most closely with lymphatic drainage, which returns plasma that has leaked out of the capillaries to the blood circulation [4]. Interstitial flow provides cells with fresh nutrients and removes waste products affecting cell–cell signaling steadily rather than periodically. Animal models, on the other hand, although presenting the same tissue microenvironment, physiology, and molecular interactions of humans, have dissimilar hormone and growth factor milieus, altered drug metabolism, and are difficult to finely tune at the cellular level [5]. These serious shortcomings emphasize the critical need to develop new in vitro biomimetic systems that better represent the in vivo human physiological conditions in effort to hasten biomedical innovation.
Microfluidic technology is emerging as a promising alternative utilized in a wide range of applications in basic and applied biomedical research. Microfluidic devices are useful tools in studying biological phenomena involving fluid flow, thin tissue culture, and cell mobility [6]; each of these aspects make microfluidic techniques attractive for studying human breast cancer, which is the most commonly occurring cancer in women and the second most common cancer overall worldwide [7][8]. Although cancer can arise from any tissue, the ductal epithelium of the breast is of prime interest, because it is the site of 20% of cancers, compared with 15% for the lung epithelium and 13% for the colon epithelium [9]. Identifying and exploiting the distinct properties of tumors is the primary focus of oncology, and microfluidic devices are innately well suited for this goal. In comparison with traditional 2D cell cultures or animal models, microfluidic devices provide smaller scales, greater reproducibility, reduced cost, a lower sample volume, and superior control over the cellular microenvironment [10]. Indeed, microfluidic devices are becoming more prevalent in academia and industry.
Although some cancers may recede into a dormant state of quiescence, many can progress towards metastatic invasion [11]. Metastatic invasion can include local invasion from the primary site, intravasation and extravasation through lymph or blood vessels, and the colonization of a secondary site within the body. Intravasation is the process where tumor cells enter the circulatory system, by invading the endothelial lining of blood or lymph vessels, to become circulating tumor cells (CTCs), enabling them to spread to remote sites in the body [12]. Extravasation is the process by which CTCs exit the circulatory system to settle in a new organ system; breast CTCs have been found to primarily extravasate into four organs, the bone, brain, liver, and lungs, where they then form secondary tumors that may be fatal [13][14]. Breast cancer metastasis is under intense investigation, and a comprehensive review of biomimetic microfluidic platforms developed for its assessment has recently been presented [15]. However, in addition to investigating the mechanisms of metastasis, microfluidic devices are also useful tools in exploring new breast cancer detection and treatment methods, with microfluidic devices having been used to test and troubleshoot various treatment modalities for development of new therapeutic strategies [16][17].
1.1. Breast Cancer Physiology
The ductal epithelium is composed of two epithelial subtypes, the basal or myoepithelium and the luminal epithelium, each of which have distinct markers [18]. The myoepithelium is in direct contact with the basement membrane, which is composed of type IV collagen and proteoglycans (Figure 1, purple cells). The luminal cells are exposed to the ductal lumen (Figure 1, yellow cells), and these cells can undergo hyperplasia and grow into the lumen to form ductal carcinoma in situ (DCIS), a non-invasive and benign, but pre-cancerous condition (Figure 1, red cells). Upon transformation, cells of either the basal or luminal epithelium can invade across the basement membrane and into the surrounding stroma or extracellular matrix, forming an invasive ductal carcinoma. Further alterations in gene expression can then lead to a metastatic cascade, whereupon cells can travel to distant organs and colonize new sites of growth and invasion.
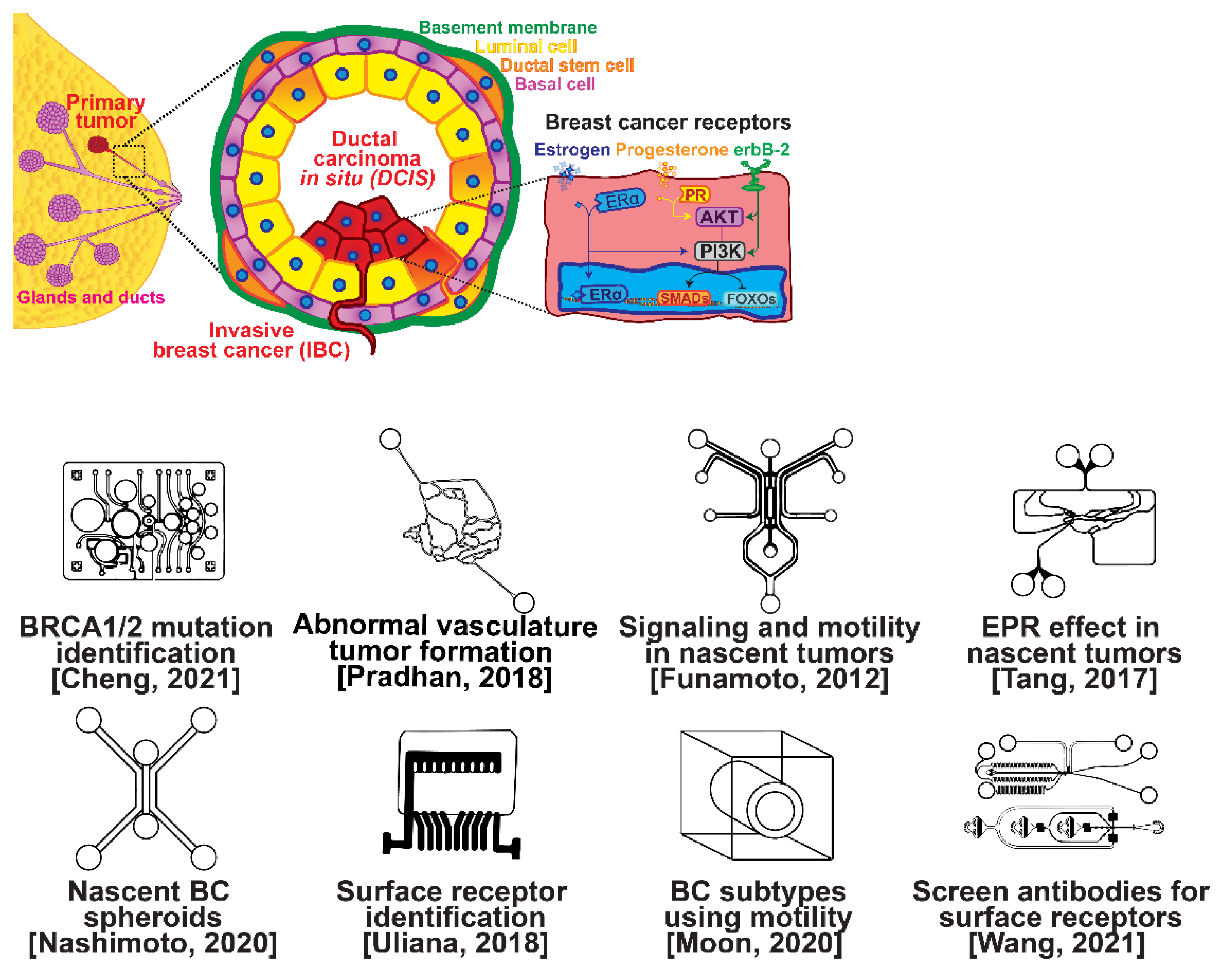
Figure 1. Selected microfluidic platforms for studying breast cancer tumor formation in ducts; normal epithelia lined by a basement membrane can proliferate locally to give rise to a tumor. Further transformation by epigenetic changes and genetic alterations leads to a carcinoma in situ, still outlined by an intact basement membrane. Three canonical surface receptors upregulated in tumor cells have been identified as distinguished biomarkers significant in cancer signaling: the estrogen receptor (ER), the progesterone receptor (PR), and the receptor tyrosine protein kinase ErbB-2, commonly known as HER2.
Several microfluidic devices have been developed to study the origin of breast cancer. A microfluidic platform was designed for screening the blood plasma of patients for DNA bearing BRCA1/2 mutations to detect ovarian-cancer-related mutations, as ovarian cancer has a similar mutational origin as breast cancer [19]. Other devices have been developed to investigate early cancer conditions. Breast cancer tumor formation and progression was modeled in a microfluidic system, recreating the pathophysiologic vasculature observed in patients, where low-perfusion physiology was found to enhance tumor progression. The vascular channels, 100 μm in height and width, were separated from the primary and secondary tumor chambers via a 100 μm wide interstitial gap, while the gap was fitted with PDMS pillars of diameter 20 μm and pillar-to-pillar spacing of 20 μm to facilitate cellular interactions [20]. The metabolism of cancer cells during tumorigenesis was explored in a similar device, demonstrating that heterogeneous adaptations to metabolic stress in nascent tumors make anti-cancer drugs targeting cancer metabolism less effective [21]. A microfluidic device was utilized to elucidate the influence of HIF-2 on early cancer motility and signaling [22]. Furthermore, the enhanced permeation and retention (EPR) effect, a controversial concept suggesting that molecules of certain sizes accumulate in tumor much more than they do in normal tissue, has been investigated in breast cancer tumorigenesis using a physiologically relevant vasculature model [23]. Finally, breast cancer spheroids were formed in a generalized breast tumor analysis microfluidic platform for early tumor physiological research and drug discovery [24].
1.2. Breast Cancer Types
Types of breast cancer include ductal carcinoma in situ (DCIS), invasive ductal carcinoma (IDC), inflammatory breast cancer (IBC), and metastatic breast cancer (MBC). Once established, breast cancer cells can be distinguished from their surrounding tissue by their distinct surface receptor expression. Three canonical surface receptors have been identified as being critical biomarkers and significant in cancer signaling—the estrogen receptor (ER), the progesterone receptor (PR), and the receptor tyrosine protein kinase ErbB-2, commonly known as HER2. These are excellent biomarkers because of the contrast in their expression within invasive breast cancer compared with healthy tissue—ER is upregulated in ~75% of IBCs, with PR upregulated in ~70% and HER2 upregulated in 10–40% [25]. However, ~10% of breast cancers do not exhibit upregulation of the ER, PR, or HER2 receptors; therefore, they are designated as triple-negative breast cancers (TNBCs), which receive signaling through a combination of less common pathways [26][27]. The ER, PR, and HER2 receptors related to breast cancers are significant in that they are highly expressed in contrast to surrounding tissue and engage with well-studied signal cascades. This enables reliable prediction of the cancer behavior, and the use of pharmacological therapy to halt the cancer from aberrant signaling expression [28][29][30][31]. Receptor expression disparity is the basis of several microfluidic platforms developed for breast cancer research, including an inexpensive and disposable microfluidic system that was constructed to enable the probing of cancer cells based on these surface receptors. The fully disposable microfluidic electrochemical array device was constructed using low-cost materials, and an inexpensive home cutter printer enabled the manufacture of dozens of devices in less than 2 h, at a cost of less than USD 0.20 in material per device [32].
2. Breast Cancer Metastasis
Metastasis is one of the hallmarks of cancer, distinguishing it from benign tumors [33]; the term is used to describe the spread of cancer from its original/primary site to a different/secondary site within the body. Unlike normal cells, cancer cells possess the ability to grow outside of the tissue in the body where they originated. Nearly all cancer types can metastasize, but whether they do metastasize depends on many factors. Metastases can occur in three ways: (i) grow directly into the tissue surrounding the tumor, (ii) travel through the lymph system to nearby or distant lymph nodes, or (iii) travel through the blood stream to distant locations. Metastasis, in general, involves invasion, intravasation, and extravasation.
2.1. Invasion Modeling
Invasion occurs as cancer cells begin to break off from the bulk of the tumor to invade its surrounding tissue as illustrated in Figure 2. As proliferation increases, cancer cells become confined, hypoxic, and metabolically starved [34][35]. These factors contribute to an epithelial-to-mesenchymal (EMT) transition within the cancer cells, changing their morphology and greatly increasing their motility [36]. Recently, E-cadherin was singled out as required for invasion in multiple models of breast cancer, which could still involve partial/hybrid EMT states [37]. Confinement has also been found to induce cancer cells to enter a highly motile amoeboid state that initiates the invasion of surrounding tissue, but invasion may also occur through slower collective and mesenchymal cell migration [38][39]. Aside from these primary stimuli, cancer cells have also been shown to become invasive or undergo EMT in response to acidity, stromal and endothelial cell crosstalk, and excess ECM density [40][41][42]. Invading breast cancer cells begin to degrade restrictive extracellular matrix components by expressing matrix metalloproteinases, such as MMP-2 and MMP-9, and similar matrix reconstruction enzymes that lead to widespread breast cancer invasion [43].
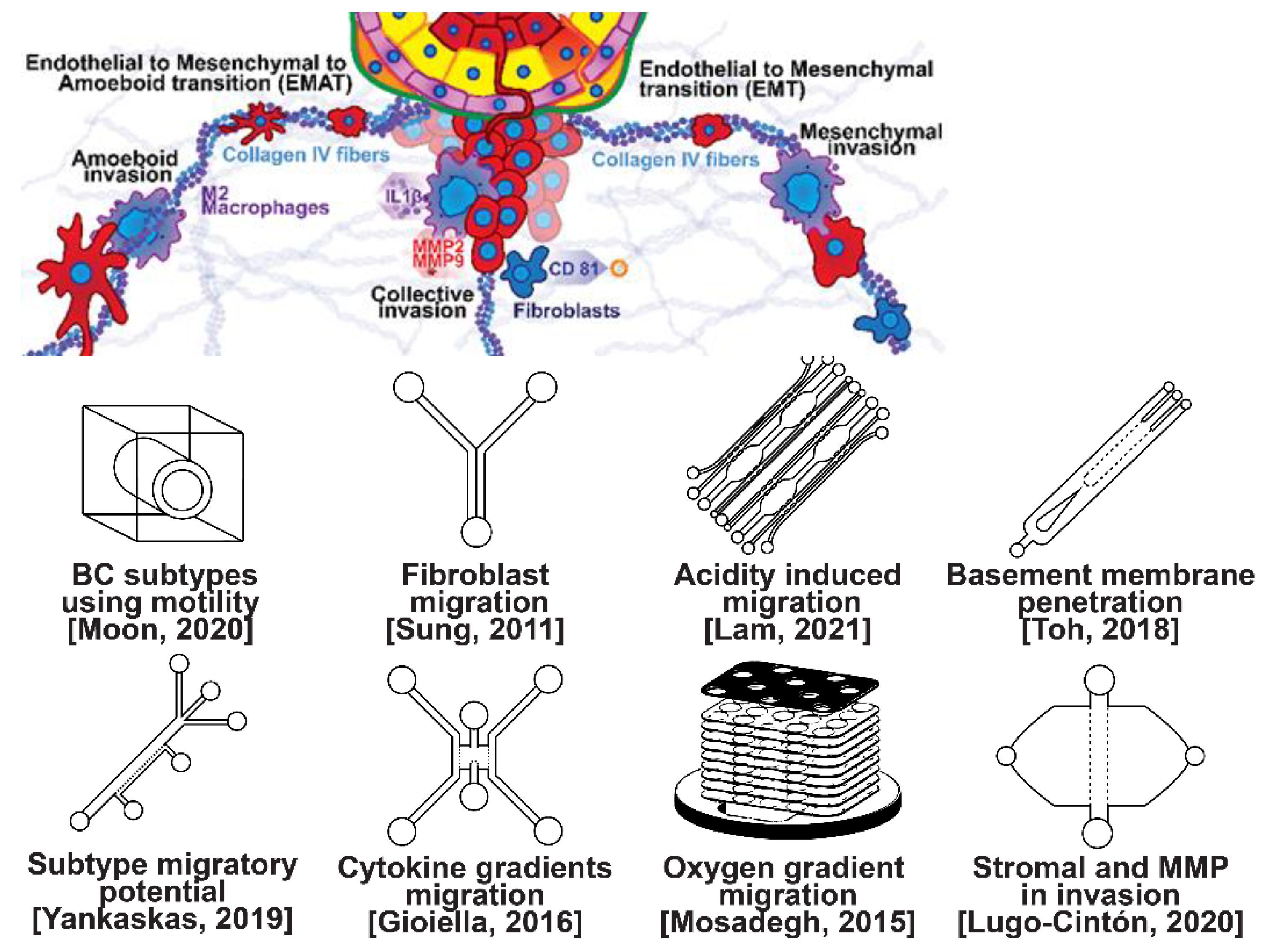
Figure 2. Selected microfluidic platforms for studying breast cancer invasion, the first stage of metastasis, which occurs as cancer cells break off from the primary breast tumor to invade the surrounding tissue. As proliferation increases, cancer cells become confined, hypoxic, and metabolically starved. These factors contribute to an epithelial-to-mesenchymal (EMT) transition within the cancer cells, changing their morphology and greatly increasing their motility. Aside from the primary stimuli of invasion: (i) highly motile amoeboid, (ii) slow collective, and (iii) mesenchymal cell migration, cancer cells have also been shown to become invasive or undergo EMT in response to acidity, stromal and endothelial cell crosstalk, healthy immune cell signaling, excess ECM density, and some forms of chemo- or radiotherapy.
Microfluidic devices allow for improved characterization of the factors related to breast cancer invasion. The migratory potential of cancer subtypes was examined in a microfluidic device based on their distinct surface receptor combinations [44]. Microfluidics has also been used to identify alternative surface receptors for further investigation, and specific subtypes were identified by observing their migratory patterns [45]. A microfluidic 3D compartmentalized system was introduced to co-culture mammary epithelial cells (MCF-DCISs) with human mammary fibroblasts (HMFs), promoting a transition from DCIS to IDC in vitro [46]. The model enabled the control of both spatial and temporal features of the microenvironment, thereby, recapitulating the in vivo environment in ways not practical with existing experimental models. Analysis of intrinsic second harmonic generation signal of collagen allowed the label-free quantitative analysis of DCIS-associated collagen remodeling. Moreover, the arrayed microchannel-based model provided a cost-effective test bed for identifying inhibitors of pathways involved in DCIS progression to IDC and for screening potential therapeutic targets. Calcium carbonate nanoparticles were found to stimulate cancer cell reprogramming to suppress tumor growth and invasion in an organ-on-a-chip system, which was developed to create a tumor microenvironment for isolating the effect of pH on tumor viability [47]. The results suggested that treating breast cancer cells (MDA-MB-231) co-cultured with fibroblasts using CaCO3 nanoparticles can restrict the aggressiveness of tumor cells without affecting the growth and behavior of the surrounding stromal cells. Breast cancer cells were observed to penetrate the duct basement membrane at the origin of invasion in a 3D microfluidic model designed to recapitulate cancer cell migration and invasion [48]. Similarly, using a microfluidic assay for the quantification of the metastatic propensity of breast cancer specimens, high invasive potential was found to be correlated with the RAS/MAPK and PI3K pathways, as previously suggested [49]. Additionally, the in vivo cytokine gradients were recreated in a microfluidic tissue culture model that permitted the visualization of various breast cancer subtypes infiltrating the surrounding tissue [50], and cancer cells were observed to preferentially invade towards regions of higher oxygen concentrations in a paper-based microfluidic device [51]. Utilizing microfluidic devices, tumor-associated macrophages (TAMs) and U-937 cells were found to promote tumor invasion [52], and breast cancer stromal cells were reported to interact with migratory tumor cells to facilitate their motility by secreting MMPs at levels that overpower anti-MMP drugs [53]. Qualitative and quantitative mapping of a large population of cell–protein interactions were carried out in a microfluidic platform; the upregulated β1 integrin in invasive cancer cells enhanced cell–ECM interaction, promoting its remodeling, and cancer cells showed strong interaction with plasma fibrinogen that may support their arrest on blood vessels [54].
2.2. Intravasation Modeling
Once able of moving through the ECM, motile breast cells have been found to follow collagen fibers that lead from their primary site to nearby blood or lymph vessels [55]. However, breast cancer cells are often incapable of penetrating the basal lamina or the endothelial cell layer surrounding the lumen of these vessels due to cadherins forming tight intercellular junctions [56][57]. This seems to be overcome with the assistance of various signaling pathways and macrophage interactions forming a microenvironment that enables cancer cells to penetrate into the bloodstream, as shown in Figure 3 [56][57][58][59][60]. Macrophages in the tumor environment display a wide phenotypic spectrum, varying from anti-tumor M1 types to pro-invasion M2 types [61]. Pro-invasion M2-like tumors express factors that lower cadherin density in vascular endothelial cells, such as the angiogenic signals TNF1α, VEGF, and EGF, as well as immune-cell recruiting signals such as the CXCL family, all of which prime the vessel for penetration [60][61][62][63][64]. Tumor cells themselves actively participate in the intravasation process through a variety of means, e.g., via the NOTCH and TGFβ1 pathways, to induce cadherin degradation and endothelial contraction, permitting extravasation [65][66][67]. Breast cancer cells secreting micro-RNA (miRNA) signals, such as miR-939, have been under investigation due to their role in stimulating endothelial permeability [68][69].

Figure 3. Selected microfluidic platforms for studying breast cancer intravasation, the process by which invading cancer cells enter a blood or lymphatic vessel allowing their passive transport to distant organs as CTCs. Once capable of moving through the ECM, motile breast cancer cells have been found to follow collagen fibers that lead from their primary site to nearby blood or lymph vessels. However, breast cancer cells are often incapable of penetrating the basal lamina or the endothelial cell layer surrounding the lumen of these vessels due to cadherins forming tight intercellular junctions. This seems to be overcome with the assistance of various signaling pathways and macrophage interactions forming a microenvironment that enables cancer cells to penetrate the vasculature.
Microfluidic devices are attractive for investigating intravasation in breast cancer because they allow precise spatial control of cell positioning. Using an engineered microfluidic migration chamber, MDA-MB-231 breast cancer cell invasion through confined microchannels was shown to induce a change in migratory phenotype [70]. To recreate the tumor–vascular interface in three dimensions, enabling precise quantification of the endothelial barrier function, a microfluidic-based assay was developed for studying the regulation of carcinoma cell intravasation by biochemical factors from the interacting cells and cellular interactions with macrophages; endothelial permeability measurements showed that signaling with macrophages via the secretion of tumor necrosis factor alpha results in endothelial barrier impairment [71]. In another co-culture study, employing a 3D cylindrical configuration coupled with confocal microscopy, tumor cells were found to significantly increase the expression of proangiogenic genes in response to co-culture with endothelial cells under low flow conditions. Using microparticle image velocimetry (μ-PIV), the flow rate was adjusted to be in the range of 260–2600 μL/min to generate a target wall shear stress of 1–10 dyn/cm2 [72]. Such a system provides a downstream molecular analysis capability which can serve as a versatile platform for elucidating the role of fluid forces on tumor–endothelial crosstalk. A biomimetic microengineering strategy was described to reconstitute 3D structural organization and the microenvironment of breast tumors in human-cell-based in vitro models. The microsystem enabled the co-culture of breast tumor spheroids with human mammary ductal epithelial cells on one side of an ECM membrane, and mammary fibroblasts on the other side of the ECM membrane, in a compartmentalized microfluidic device to replicate the microarchitecture of breast ductal carcinoma in situ (DCIS) [73]. A microfluidic co-culture system was presented for the establishment of mild, moderate, and severe cancer models to study cancer cell migration. The density of the cancer cells was reported to determine the probability of the occurrence of metastatic cells as well as their velocity, with the increase in the migration velocity of MDA-MB-231 cells co-cultured with HMEpiC cells found to be proportional to the increased secretion of IL-6 [74]. Trans-endothelial migration has also been explored using a high-throughput multi-channel microfluidic device [75]. A 3D microfluidic platform comprising concentric three-layer cell-laden hydrogels was developed for the simultaneous investigation of breast cancer cell invasion and intravasation as well as vasculature maturation influenced by tumor–vascular crosstalk. It was demonstrated that the presence of a spontaneously formed vasculature enhanced MDA-MB-231 invasion into the 3D stroma. The invading cancer cells significantly reduced vessel diameter while increasing permeability, and major signaling cytokines involved in tumor–vascular crosstalk governing cancer cell invasion and intravasation were identified [76]. To dynamically observe tumor progression, including cell migration, angiogenesis, and tumor cell intravasation, a microfluidic platform was realized for mimicking biological mass transport near the arterial end of a capillary in the tumor microenvironment [77]. However, most in vitro metastasis models favor investigating blood-vessel-based metastasis pathways; thus, the understanding of lymphatic metastasis is limited, which is also closely related to the inflammatory system. To understand the effects of inflammatory cytokines in lymphatic metastasis, a three-channel microfluidic system was constructed to mimic the lymph vessel–tissue–blood vessel (LTB) structure; each channel was about 100 μm in width. Matrigel was injected into the middle channel to mimic the tissue, while human umbilical vein endothelial and human lymphatic endothelial cell layers were formed in the side channels to reconstitute blood and lymph vessels, respectively. The exposure of different subtypes of breast cancer cells to an inflammatory cytokine, interleukin 6 (IL-6), induced epithelial–mesenchymal transition and enhanced tissue invasion. Similar LTB chips could be applied to analyze the intercellular communication in the tumor microenvironment under various extracellular stimuli such as inflammatory cytokines, stromal reactions, hypoxia, and nutrient deficiency [78].
2.3. Extravasation Modeling
Once within a lymph or blood vessel, tumor cells become circulating tumor cells (CTCs) that are targeted by different immune cells to varying extents [79]. Individual CTCs resist immune system attacks through a myriad of mechanisms, such as the degradation of apoptotic receptors such as TRAIL, and the expression of attack-arresting surface markers such as CD47 and PD-L1 [80][81][82]. However, individual CTCs may undergo apoptosis under the influence of immune cytokines and fluid shear forces, and undergo anoikis upon loss of attachment to the extracellular matrix (ECM) and neighboring cells due to a lack of fibronectin mediation [83][84]. CTCs that aggregate through CD-44 cohesion have been shown to be more resilient, particularly when the aggregates include platelets and neutrophils which disguise them [85][86]. Heterogenous cell aggregates also lead to CTC proliferation through IL-1β and IL-6 crosstalk [87]. CTCs spread with circulation to remote sites and, subsequently, they can extravasate from the vascular system through blood vessel walls into the surrounding tissue forming tumors at host organs, as shown in Figure 4. The site of CTC arrest is known as the secondary site and is found to occur primarily in selective organs. This targeted arrest is known as organotropism and is described in Figure 5, with breast cancer secondary sites appearing mainly in the bone, brain, liver, and lungs, in addition to the axillary lymph nodes, which are diagnostic for metastatic disease in ~97% of patients [13][14][88]. Organotropism is presumed to originate from the primary tumor secreting context-dependent signals to distal sites that sensitize them to receive CTCs which, in cooperation with neutrophils, seek the pre-conditioned sites [89][90][91]. Certain signals have been implicated with specific organotrophic targets, such as TGFβ and COX2 for the lungs, SRC-dependent pathways for bone, and IGF1 for the brain [92][93][94]. mi-RNAs have also been identified as significant in targeted organotropism, with miR-16/148a for the lungs and miR-127/197/222/223 for bone [89][90][91]. Tumor exosomes may be involved in organotropism; tumor-derived exosome uptake by organ-specific cells was observed to prepare the pre-metastatic niche, and treatment with exosomes from lung-tropic models redirected the metastasis of bone-tropic tumor cells. Moreover, exosome proteomics revealed distinct integrin expression patterns, in which the exosomal integrins α6β4 and α6β1 were associated with lung metastasis, whereas exosomal integrin αvβ5 was linked to liver metastasis. Thus, targeting integrins α6β4 and αvβ5 decreased exosome uptake as well as lung and liver metastasis, respectively. Exosome integrin uptake by resident cells was found to activate Src phosphorylation and pro-inflammatory S100 gene expression [95]. Outside of chaperoned extravasation, CTCs and CTC clusters may arrest along the vasculature through weak CD-44 and integrin αvβ3 binding, and are then reinforced with stronger fibronectin and integrin α5β1 bonds over time [96]. Once the CTCs affix to their target organ, a similar process to intravasation then occurs, where CTCs excrete signals with neutrophils to permeabilize endothelial cells for infiltration [97]. Trans-endothelial migration enables cancer cells to embed between endothelial cells, mesenchymal stem cells (MSCs), and vascular pericytes [98]. This perivascular niche produces supportive signals such as PI3K, and is a main focus of anti-cancer research [99][100]. Pericytes also play a significant role in breast cancer extravasation, and have been shown to respond to tumor-derived signals by performing an embryogenesis-derived program of angiotrophic migration and fibroblast differentiation, enhancing tumor extravasation and promoting pericyte mimicry (PM) in cancer cells [101][102][103]. Pericyte- and MSC-expressed MCAM/CD146 were reported to alter the expression of the estrogen receptors ErbB3 and ErbB4, resulting in an increase in chemoresistance, EMT, and a worse prognosis, in contrast to previous findings [104][105][106][107]. Confinement within the perivascular niche has been found to induce cancer cells to enter a motile amoeboid morphology that initiates the invasion of surrounding tissue, although invasion may also occur through slower collective and mesenchymal cell migration modes [38][39]. Signals such as EGF, BMP2/7, AKT, and WNT may then reverse EMT by producing a mesenchymal-to-epithelial transition (MET) or amoeboid-to-epithelial transition (AET), indicated by a FGFR2b/FGFR2 switch that leads to migratory arrest and tumorigenesis [108][109].
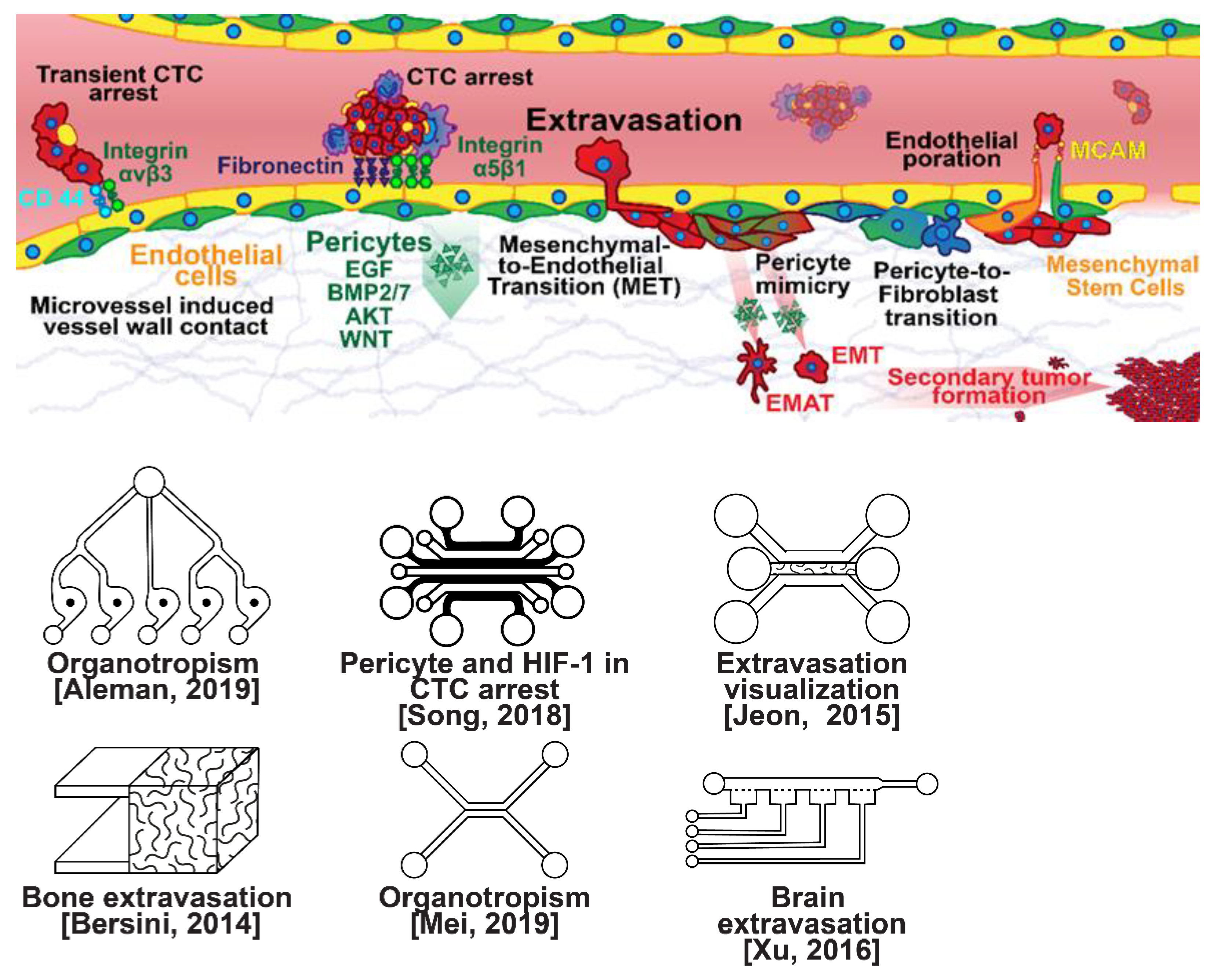
Figure 4. Selected microfluidic platforms for studying breast cancer extravasation, the process by which CTCs exit the vasculature in remote host organs. Individual CTCs resist immune system attacks through a myriad of mechanisms, such as the degradation of apoptotic receptors and the expression of attack-arresting surface markers. However, individual CTCs may undergo apoptosis under the influence of immune cytokines and fluid shear forces, and undergo anoikis upon loss of attachment to the extracellular matrix (ECM) and neighboring cells due to a lack of fibronectin mediation. CTCs that aggregate have been shown to be more resilient, particularly when the aggregates include platelets and neutrophils which disguise them. At remote sites, solitary carcinoma cells can extravasate through either endothelial poration or pericyte-mediated recruitment; they then may remain solitary (micrometastasis) or form a new secondary tumor through EMT (macrometastasis).
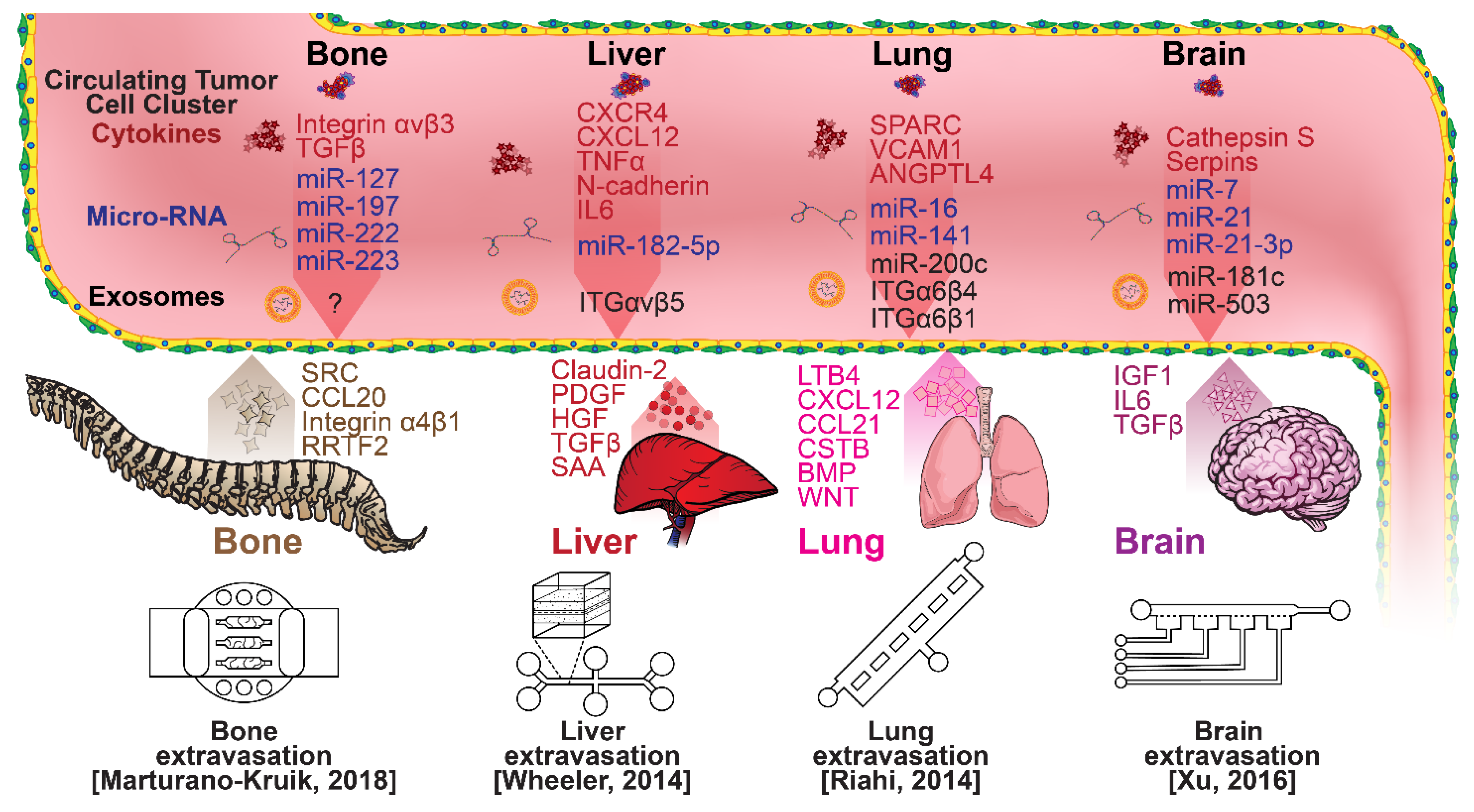
Figure 5. Selected microfluidic platforms for studying breast cancer organotropism, which is the organ-specific extravasation of CTCs involving: (i) the release of pro-inflammatory cytokines into circulation by the primary tumor, (ii) the upregulation of endothelial adhesion molecules at secondary sites induced by the cytokines, (iii) CTCs adhering to and migrating through endothelial cells and proliferating. Breast CTCs have been found to primarily extravasate into the bone, liver, lungs, and brain, where they form secondary tumors that may be fatal.
Microfluidic devices could provide a powerful tool for improving the scientific understanding of CTC behavior, organotropism, and extravasation. A microfluidic method was introduced for the integrated capture, isolation, and analysis of membrane markers, as well as the quantification of proteins secreted by single CTCs and CTC clusters. The proposed platform was tested with multiple breast cancer cell lines spiked into human blood and mouse-model-derived CTCs. The quantified secretion level of granulocyte growth-stimulating factor (G-CSF), which is involved in neutrophil recruitment, was found to be highly expressed across cancer cell lines. Incorporating barcoded magnetic beads, this platform can be adapted for multiplexed analysis enabling comprehensive functional CTC profiling [110]. A thorough investigation of CTC–neutrophil adhesion was conducted using droplet formation techniques, which showed that CTC–neutrophil aggregates upregulated the expression of VCAM-1, E-cadherin, and macrophage recruitment cytokines such as CCL4/24/22 and PPBP [111]. Utilizing a commercially available hepatic microphysiologic system (LiverChip, CN Bio Innovations Limited, Oxford, UK), the liver system was established as a relevant microfluidic model for the study of breast cancer metastasis [112]. A microfluidic system was developed and characterized for the in vitro systematic studies of organ-specific extravasation of CTCs. The system recapitulated the two major aspects of the in vivo extravasation microenvironment: local signaling chemokine gradients in a vessel lined up with an endothelial monolayer. The system was utilized to demonstrate the extravasation of CXCR4-expressing MDA-MB-231 cancer cells, across a confluent HUVEC monolayer, in the presence of CXCL12 chemokine gradients. Consistent with the hypothesis of organ-specific extravasation, control experiments were presented to substantiate the observation that the MDA-MB-231 cell migration was due to controlled chemotaxis rather than a random process [113]. A multi-site metastasis-on-a-chip microphysiological system was described for assessing the metastatic preference of cancer cells. The device housed multiple bioengineered 3D organoids established by a 3D photopatterning technique employing extracellular-matrix-derived hydrogel biomaterials. Under recirculating fluid flow, tumor cells grew in the primary site, entered circulation, and preferentially homed to specific organ constructs. The platform can be implemented to better understand the mechanisms underlying metastasis and, perhaps, leading to the identification of targets for intervention [114]. The impact of hypoxia, a common feature of the tumor microenvironment, on the extravasation potential of breast cell lines, was studied in a 3D microvascular network model. Using HIF-1α knock-down cell lines, the importance of HIF-1α in the transmigration ability of human breast cell lines was validated. Under hypoxic conditions, the HIF-1α protein level was increased, and coincided with changes in cell morphology, viability, and an elevated metastatic potential; these changes were accompanied with an increase in the rate of extravasation compared with normoxia (21% O2). Such a microfluidic model can be a reliable in vitro tool for systematically interrogating individual factors and their accompanying downstream effects, which may otherwise be difficult to study in complex tumor tissues [115]. A physiologically relevant vascularized bone matrix to model CTC extravasation into the perivascular niche was created in a similar device, which was then used to demonstrate the anti-metastatic role of interstitial flow [116]. Other projects on the perivascular niche added to the investigation of extravasation by providing alternative designs for imaging and quantifying CTC extravasation [116][117][118][119]. A dynamic in vivo-like 3D microfluidic system replicating key structural, functional, and mechanical properties of the blood–brain barrier (BBB) in vivo was constructed to probe metastatic brain tumors. Multiple factors in this organotypic BBB model work synergistically to accentuate BBB-specific attributes with the complex microenvironment reproduced via physical cell–cell interaction and vascular mechanical cues. The interactions between cancer cells and astrocytes in the BBB microenvironment seemed to affect the ability of malignant brain tumors to traverse between brain and vascular compartments. The model offers the capability of examining brain metastasis of human breast cancer cells and their therapeutic responses to chemotherapy. Furthermore, the quantification of spatially resolved barrier functions exists within a single assay, providing a versatile platform for pharmaceutical development, drug testing, and neuroscientific research [120].
This entry is adapted from the peer-reviewed paper 10.3390/mi13020152
References
- Thomas, R.S.; Black, M.B.; Li, L.; Healy, E.; Chu, T.-M.; Bao, W.; Andersen, M.E.; Wolfinger, R.D. A Comprehensive Statistical Analysis of Predicting In Vivo Hazard Using High-Throughput In Vitro Screening. Toxicol. Sci. 2012, 128, 398–417.
- Selimović, Š.; Dokmeci, M.R.; Khademhosseini, A. Organs-on-a-chip for drug discovery. Curr. Opin. Pharmacol. 2013, 13, 829–833.
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200.
- Swartz, M.A.; Fleury, M.E. Interstitial Flow and Its Effects in Soft Tissues. Annu. Rev. Biomed. Eng. 2007, 9, 229–256.
- Holen, I.; Speirs, V.; Morrissey, B.; Blyth, K. In vivo models in breast cancer research: Progress, challenges and future directions. Dis. Model. Mech. 2017, 10, 359–371.
- Halldorsson, S.; Lucumi, E.; Gómez-Sjöberg, R.; Fleming, R.M.T. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens. Bioelectron. 2015, 63, 218–231.
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789.
- Azamjah, N.; Soltan-Zadeh, Y.; Zayeri, F. Global Trend of Breast Cancer Mortality Rate: A 25-Year Study. Asian Pac. J. Cancer Prev. 2019, 20, 2015–2020.
- Dong, M.; Cioffi, G.; Wang, J.; Waite, K.; Ostrom, Q.; Kruchko, C.; Lathia, J.; Rubin, J.; Berens, M.; Connor, J.; et al. Sex Differences in Cancer Incidence and Survival: A Pan-Cancer Analysis. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1389–1397.
- Franzen, N.; van Harten, W.H.; Retèl, V.P.; Loskill, P.; van den Eijnden-van Raaij, J.; IJzerman, M. Impact of organ-on-a-chip technology on pharmaceutical R&D costs. Drug Discov. Today 2019, 24, 1720–1724.
- Chen, W.; Dong, J.; Haiech, J.; Kilhoffer, M.-C.; Zeniou, M. Cancer Stem Cell Quiescence and Plasticity as Major Challenges in Cancer Therapy. Stem Cells Int. 2016, 2016, 1–16.
- Strilic, B.; Offermanns, S. Intravascular Survival and Extravasation of Tumor Cells. Cancer Cell 2017, 32, 282–293.
- Disibio, G.; French, S.W. Metastatic Patterns of Cancers: Results From a Large Autopsy Study. Arch. Pathol. Lab. Med. 2008, 132, 931–939.
- Soni, A.; Ren, Z.; Hameed, O.; Chanda, D.; Morgan, C.J.; Siegal, G.P.; Wei, S. Breast Cancer Subtypes Predispose the Site of Distant Metastases. Am. J. Clin. Pathol. 2015, 143, 471–478.
- Sigdel, I.; Gupta, N.; Faizee, F.; Khare, V.M.; Tiwari, A.K.; Tang, Y. Biomimetic Microfluidic Platforms for the Assessment of Breast Cancer Metastasis. Front. Bioeng. Biotechnol. 2021, 9, 633671.
- Krol, I.; Schwab, F.D.; Carbone, R.; Ritter, M.; Picocci, S.; De Marni, M.L.; Stepien, G.; Franchi, G.M.; Zanardi, A.; Rissoglio, M.D.; et al. Detection of clustered circulating tumour cells in early breast cancer. Br. J. Cancer 2021, 125, 23–27.
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int. J. Oncol. 2019, 13.
- Saeki, K.; Chang, G.; Kanaya, N.; Wu, X.; Wang, J.; Bernal, L.; Ha, D.; Neuhausen, S.L.; Chen, S. Mammary cell gene expression atlas links epithelial cell remodeling events to breast carcinogenesis. Commun. Biol. 2021, 4, 660.
- Cheng, Y.-H.; Wang, C.-H.; Hsu, K.-F.; Lee, G.-B. An Integrated Microfluidic Platform for Detecting BRCA1/BRCA2 Gene Mutation and Risk Assessment of Ovarian Cancer. In Proceedings of the 2021 21st International Conference on Solid-State Sensors, Actuators and Microsystems (Transducers), Orlando, FL, USA, 20–24 June 2021; pp. 1024–1027.
- Pradhan, S.; Smith, A.M.; Garson, C.J.; Hassani, I.; Seeto, W.J.; Pant, K.; Arnold, R.D.; Prabhakarpandian, B.; Lipke, E.A. A Microvascularized Tumor-mimetic Platform for Assessing Anti-cancer Drug Efficacy. Sci. Rep. 2018, 8, 3171.
- Ayuso, J.M.; Gillette, A.; Lugo-Cintrón, K.; Acevedo-Acevedo, S.; Gomez, I.; Morgan, M.; Heaster, T.; Wisinski, K.B.; Palecek, S.P.; Skala, M.C.; et al. Organotypic microfluidic breast cancer model reveals starvation-induced spatial-temporal metabolic adaptations. EBioMedicine 2018, 37, 144–157.
- Funamoto, K.; Zervantonakis, I.K.; Liu, Y.; Ochs, C.J.; Kim, C.; Kamm, R.D. A novel microfluidic platform for high-resolution imaging of a three-dimensional cell culture under a controlled hypoxic environment. Lab. Chip 2012, 12, 4855.
- Tang, Y.; Soroush, F.; Sheffield, J.B.; Wang, B.; Prabhakarpandian, B.; Kiani, M.F. A Biomimetic Microfluidic Tumor Microenvironment Platform Mimicking the EPR Effect for Rapid Screening of Drug Delivery Systems. Sci. Rep. 2017, 7, 9359.
- Nashimoto, Y.; Okada, R.; Hanada, S.; Arima, Y.; Nishiyama, K.; Miura, T.; Yokokawa, R. Vascularized cancer on a chip: The effect of perfusion on growth and drug delivery of tumor spheroid. Biomaterials 2020, 229, 119547.
- Viale, G.; Regan, M.M.; Maiorano, E.; Mastropasqua, M.G.; Dell’Orto, P.; Rasmussen, B.B.; Raffoul, J.; Neven, P.; Orosz, Z.; Braye, S.; et al. Prognostic and Predictive Value of Centrally Reviewed Expression of Estrogen and Progesterone Receptors in a Randomized Trial Comparing Letrozole and Tamoxifen Adjuvant Therapy for Postmenopausal Early Breast Cancer: BIG 1-98. J. Clin. Oncol. 2007, 25, 3846–3852.
- Huang, M.; Wu, J.; Ling, R.; Li, N. Quadruple negative breast cancer. Breast Cancer 2020, 27, 527–533.
- Siddharth, S.; Sharma, D. Racial Disparity and Triple-Negative Breast Cancer in African-American Women: A Multifaceted Affair between Obesity, Biology, and Socioeconomic Determinants. Cancers 2018, 10, 514.
- Iwamoto, T.; Booser, D.; Valero, V.; Murray, J.L.; Koenig, K.; Esteva, F.J.; Ueno, N.T.; Zhang, J.; Shi, W.; Qi, Y.; et al. Estrogen Receptor (ER) mRNA and ER-Related Gene Expression in Breast Cancers That Are 1% to 10% ER-Positive by Immunohistochemistry. J. Clin. Oncol. 2012, 30, 729–734.
- Ross, D.S.; Zehir, A.; Brogi, E.; Konno, F.; Krystel-Whittemore, M.; Edelweiss, M.; Berger, M.F.; Toy, W.; Chandarlapaty, S.; Razavi, P.; et al. Immunohistochemical analysis of estrogen receptor in breast cancer with ESR1 mutations detected by hybrid capture-based next-generation sequencing. Mod. Pathol. 2019, 32, 81–87.
- Kumar, M.; Sahu, R.K.; Goyal, A.; Sharma, S.; Kaur, N.; Mehrotra, R.; Singh, U.R.; Hedau, S. BRCA1 Promoter Methylation and Expression-Associations with ER+, PR+ and HER2+ Subtypes of Breast Carcinoma. Asian Pac. J. Cancer Prev. 2017, 18, 3293.
- Verdu, M.; Trias, I.; Roman, R.; Rodon, N.; Pubill, C.; Arraiza, N.; Martinez, B.; Garcia-Pelaez, B.; Serrano, T.; Puig, X. Cross-reactivity of EGFR Mutation-specific Immunohistochemistry Assay in HER2-positive Tumors. Appl. Immunohistochem. Mol. Morphol. 2015, 23, 565–570.
- Uliana, C.V.; Peverari, C.R.; Afonso, A.S.; Cominetti, M.R.; Faria, R.C. Fully disposable microfluidic electrochemical device for detection of estrogen receptor alpha breast cancer biomarker. Biosens. Bioelectron. 2018, 99, 156–162.
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674.
- Hoffmann, C.; Mao, X.; Brown-Clay, J.; Moreau, F.; Al Absi, A.; Wurzer, H.; Sousa, B.; Schmitt, F.; Berchem, G.; Janji, B.; et al. Hypoxia promotes breast cancer cell invasion through HIF-1α-mediated up-regulation of the invadopodial actin bundling protein CSRP2. Nat. Sci. Rep. 2018, 8, 10191.
- Marín-Hernández, Á.; Gallardo-Pérez, J.C.; Hernández-Reséndiz, I.; Del Mazo-Monsalvo, I.; Robledo-Cadena, D.X.; Moreno-Sánchez, R.; Rodríguez-Enríquez, S. Hypoglycemia Enhances Epithelial-Mesenchymal Transition and Invasiveness, and Restrains the Warburg Phenotype, in Hypoxic HeLa Cell Cultures and Microspheroids: Hypoglycemia Restrains the Warburg Phenotype in Hypoxic Cancer Cells. J. Cell. Physiol. 2017, 232, 1346–1359.
- Wang, Y.; Zhou, B.P. Epithelial-mesenchymal transition in breast cancer progression and metastasis. Chin. J. Cancer 2011, 30, 603–611.
- Padmanaban, V.; Krol, I.; Suhail, Y.; Szczerba, B.M.; Aceto, N.; Bader, J.S.; Ewald, A.J. E-cadherin is required for metastasis in multiple models of breast cancer. Nature 2019, 573, 439–444.
- Liu, Y.-J.; Le Berre, M.; Lautenschlaeger, F.; Maiuri, P.; Callan-Jones, A.; Heuzé, M.; Takaki, T.; Voituriez, R.; Piel, M. Confinement and Low Adhesion Induce Fast Amoeboid Migration of Slow Mesenchymal Cells. Cell 2015, 160, 659–672.
- Wu, J.; Jiang, J.; Chen, B.; Wang, K.; Tang, Y.; Liang, X. Plasticity of cancer cell invasion: Patterns and mechanisms. Transl. Oncol. 2021, 14, 100899.
- Estrella, V.; Chen, T.; Lloyd, M.; Wojtkowiak, J.; Cornnell, H.H.; Ibrahim-Hashim, A.; Bailey, K.; Balagurunathan, Y.; Rothberg, J.M.; Sloane, B.F.; et al. Acidity Generated by the Tumor Microenvironment Drives Local Invasion. Cancer Res. 2013, 73, 1524–1535.
- Hanley, C.J.; Henriet, E.; Sirka, O.K.; Thomas, G.J.; Ewald, A.J. Tumor-Resident Stromal Cells Promote Breast Cancer Invasion through Regulation of the Basal Phenotype. Mol. Cancer Res. 2020, 18, 1615–1622.
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 2020, 11, 5120.
- Li, H.; Qiu, Z.; Li, F.; Wang, C. The relationship between MMP-2 and MMP-9 expression levels with breast cancer incidence and prognosis. Oncol. Lett. 2017, 14, 5865–5870.
- Moon, H.; Ospina-Muñoz, N.; Noe-Kim, V.; Yang, Y.; Elzey, B.D.; Konieczny, S.F.; Han, B. Subtype-specific characterization of breast cancer invasion using a microfluidic tumor platform. PLoS ONE 2020, 15, e0234012.
- Aw Yong, K.M.; Ulintz, P.J.; Caceres, S.; Cheng, X.; Bao, L.; Wu, Z.; Jiagge, E.M.; Merajver, S.D. Heterogeneity at the invasion front of triple negative breast cancer cells. Sci. Rep. 2020, 10, 5781.
- Sung, K.E.; Yang, N.; Pehlke, C.; Keely, P.J.; Eliceiri, K.W.; Friedl, A.; Beebe, D.J. Transition to invasion in breast cancer: A microfluidic in vitro model enables examination of spatial and temporal effects. Integr Biol 2011, 3, 439–450.
- Lam, S.F.; Bishop, K.W.; Mintz, R.; Fang, L.; Achilefu, S. Calcium carbonate nanoparticles stimulate cancer cell reprogramming to suppress tumor growth and invasion in an organ-on-a-chip system. Sci. Rep. 2021, 11, 9246.
- Toh, Y.-C.; Raja, A.; Yu, H.; van Noort, D. A 3D Microfluidic Model to Recapitulate Cancer Cell Migration and Invasion. Bioengineering 2018, 5, 29.
- Yankaskas, C.L.; Thompson, K.N.; Paul, C.D.; Vitolo, M.I.; Mistriotis, P.; Mahendra, A.; Bajpai, V.K.; Shea, D.J.; Manto, K.M.; Chai, A.C.; et al. A microfluidic assay for the quantification of the metastatic propensity of breast cancer specimens. Nat. Biomed. Eng. 2019, 3, 452–465.
- Gioiella, F.; Urciuolo, F.; Imparato, G.; Brancato, V.; Netti, P.A. An Engineered Breast Cancer Model on a Chip to Replicate ECM-Activation In Vitro during Tumor Progression. Adv. Healthc. Mater. 2016, 5, 3074–3084.
- Mosadegh, B.; Lockett, M.R.; Minn, K.T.; Simon, K.A.; Gilbert, K.; Hillier, S.; Newsome, D.; Li, H.; Hall, A.B.; Boucher, D.M.; et al. A paper-based invasion assay: Assessing chemotaxis of cancer cells in gradients of oxygen. Biomaterials 2015, 52, 262–271.
- Mi, S.; Liu, Z.; Du, Z.; Yi, X.; Sun, W. Three-dimensional microfluidic tumor-macrophage system for breast cancer cell invasion. Biotechnol. Bioeng. 2019, 116, 1731–1741.
- Lugo-Cintrón, K.M.; Gong, M.M.; Ayuso, J.M.; Tomko, L.A.; Beebe, D.J.; Virumbrales-Muñoz, M.; Ponik, S.M. Breast Fibroblasts and ECM Components Modulate Breast Cancer Cell Migration through the Secretion of MMPs in a 3D Microfluidic Co-Culture Model. Cancers 2020, 12, 1173.
- Liu, Z.; Han, X.; Chen, R.; Zhang, K.; Li, Y.; Fruge, S.; Jang, J.h.; Ma, Y.; Qin, L. Microfluidic Mapping of Cancer Cell–Protein Binding Interaction. ACS Appl. Mater. Interfaces 2017, 9, 22143–22148.
- Han, W.; Chen, S.; Yuan, W.; Fan, Q.; Tian, J.; Wang, X.; Chen, L.; Zhang, X.; Wei, W.; Liu, R.; et al. Oriented collagen fibers direct tumor cell intravasation. Proc. Natl. Acad. Sci. USA 2016, 113, 11208–11213.
- Ginter, P.S.; Karagiannis, G.S.; Entenberg, D.; Lin, Y.; Condeelis, J.; Jones, J.; Oktay, M.H. Tumor Microenvironment of Metastasis (TMEM) Doorways Are Restricted to the Blood Vessel Endothelium in Both Primary Breast Cancers and Their Lymph Node Metastases. Cancers 2019, 11, 1507.
- Vestweber, D.; Winderlich, M.; Cagna, G.; Nottebaum, A.F. Cell adhesion dynamics at endothelial junctions: VE-cadherin as a major player. Trends Cell Biol. 2009, 19, 8–15.
- Sanchez, L.R.; Borriello, L.; Entenberg, D.; Condeelis, J.S.; Oktay, M.H.; Karagiannis, G.S. The emerging roles of macrophages in cancer metastasis and response to chemotherapy. J. Leukoc. Biol. 2019, 106, 259–274.
- Qian, B.; Deng, Y.; Im, J.H.; Muschel, R.J.; Zou, Y.; Li, J.; Lang, R.A.; Pollard, J.W. A Distinct Macrophage Population Mediates Metastatic Breast Cancer Cell Extravasation, Establishment and Growth. PLoS ONE 2009, 4, e6562.
- Linde, N.; Casanova-Acebes, M.; Sosa, M.S.; Mortha, A.; Rahman, A.; Farias, E.; Harper, K.; Tardio, E.; Reyes Torres, I.; Jones, J.; et al. Macrophages orchestrate breast cancer early dissemination and metastasis. Nat. Commun. 2018, 9, 21.
- Oshi, M.; Tokumaru, Y.; Asaoka, M.; Yan, L.; Satyananda, V.; Matsuyama, R.; Matsuhashi, N.; Futamura, M.; Ishikawa, T.; Yoshida, K.; et al. M1 Macrophage and M1/M2 ratio defined by transcriptomic signatures resemble only part of their conventional clinical characteristics in breast cancer. Sci. Rep. 2020, 10, 16554.
- Arwert, E.N.; Harney, A.S.; Entenberg, D.; Wang, Y.; Sahai, E.; Pollard, J.W.; Condeelis, J.S. A Unidirectional Transition from Migratory to Perivascular Macrophage Is Required for Tumor Cell Intravasation. Cell Rep. 2018, 23, 1239–1248.
- Munir, M.T.; Kay, M.K.; Kang, M.H.; Rahman, M.M.; Al-Harrasi, A.; Choudhury, M.; Moustaid-Moussa, N.; Hussain, F.; Rahman, S.M. Tumor-Associated Macrophages as Multifaceted Regulators of Breast Tumor Growth. Int. J. Mol. Sci. 2021, 22, 6526.
- Sainson, R.C.A.; Johnston, D.A.; Chu, H.C.; Holderfield, M.T.; Nakatsu, M.N.; Crampton, S.P.; Davis, J.; Conn, E.; Hughes, C.C.W. TNF primes endothelial cells for angiogenic sprouting by inducing a tip cell phenotype. Blood 2008, 111, 4997–5007.
- Yvette Drabsch & Peter ten Dijk TGF-β Signaling in Breast Cancer Cell Invasion and Bone Metastasis. J Mammary Gland Biol Neoplasia 2011, 16, 97–101.
- Rodriguez-Vita, J.; Fischer, A. Notch signaling facilitates crossing of endothelial barriers by tumor cells. Mol. Cell. Oncol. 2017, 4, e1311828.
- Lopes-Bastos, B.M.; Jiang, W.G.; Cai, J. Tumour–Endothelial Cell Communications: Important and Indispensable Mediators of Tumour Angiogenesis. Anticancer Res. 2016, 36, 1119–1126.
- Di Modica, M.; Regondi, V.; Sandri, M.; Iorio, M.V.; Zanetti, A.; Tagliabue, E.; Casalini, P.; Triulzi, T. Breast cancer-secreted miR-939 downregulates VE-cadherin and destroys the barrier function of endothelial monolayers. Cancer Lett. 2017, 384, 94–100.
- Kanchan, R.K.; Siddiqui, J.A.; Mahapatra, S.; Batra, S.K.; Nasser, M.W. microRNAs Orchestrate Pathophysiology of Breast Cancer Brain Metastasis: Advances in Therapy. Mol. Cancer 2020, 19, 29.
- Balzer, E.M.; Tong, Z.; Paul, C.D.; Hung, W.; Stroka, K.M.; Boggs, A.E.; Martin, S.S.; Konstantopoulos, K. Physical confinement alters tumor cell adhesion and migration phenotypes. FASEB J. 2012, 26, 4045–4056.
- Zervantonakis, I.K.; Hughes-Alford, S.K.; Charest, J.L.; Condeelis, J.S.; Gertler, F.B.; Kamm, R.D. Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Proc. Natl. Acad. Sci. USA 2012, 109, 13515–13520.
- Buchanan, C.F.; Voigt, E.E.; Szot, C.S.; Freeman, J.W.; Vlachos, P.P.; Rylander, M.N. Three-Dimensional Microfluidic Collagen Hydrogels for Investigating Flow-Mediated Tumor-Endothelial Signaling and Vascular Organization. Tissue Eng. Part C Methods 2014, 20, 64–75.
- Choi, Y.; Hyun, E.; Seo, J.; Blundell, C.; Kim, H.C.; Lee, E.; Lee, S.H.; Moon, A.; Moon, W.K.; Huh, D. A microengineered pathophysiological model of early-stage breast cancer. Lab. Chip 2015, 15, 3350–3357.
- Mi, S.; Du, Z.; Xu, Y.; Wu, Z.; Qian, X.; Zhang, M.; Sun, W. Microfluidic co-culture system for cancer migratory analysis and anti-metastatic drugs screening. Sci. Rep. 2016, 6, 35544.
- Cui, X.; Guo, W.; Sun, Y.; Sun, B.; Hu, S.; Sun, D.; Lam, R.H.W. A microfluidic device for isolation and characterization of transendothelial migrating cancer cells. Biomicrofluidics 2017, 11, 014105.
- Nagaraju, S.; Truong, D.; Mouneimne, G.; Nikkhah, M. Microfluidic Tumor–Vascular Model to Study Breast Cancer Cell Invasion and Intravasation. Adv. Healthc. Mater. 2018, 7, 1701257.
- Shirure, V.S.; Bi, Y.; Curtis, M.B.; Lezia, A.; Goedegebuure, M.M.; Goedegebuure, S.P.; Aft, R.; Fields, R.C.; George, S.C. Tumor-on-a-chip platform to investigate progression and drug sensitivity in cell lines and patient-derived organoids. Lab. Chip 2018, 18, 3687–3702.
- Cho, H.-Y.; Choi, J.-H.; Kim, K.-J.; Shin, M.; Choi, J.-W. Microfluidic System to Analyze the Effects of Interleukin 6 on Lymphatic Breast Cancer Metastasis. Front. Bioeng. Biotechnol. 2021, 8, 611802.
- Leone, K.; Poggiana, C.; Zamarchi, R. The Interplay between Circulating Tumor Cells and the Immune System: From Immune Escape to Cancer Immunotherapy. Diagnostics 2018, 8, 59.
- Zhao, H.; Wang, J.; Kong, X.; Li, E.; Liu, Y.; Du, X.; Kang, Z.; Tang, Y.; Kuang, Y.; Yang, Z.; et al. CD47 Promotes Tumor Invasion and Metastasis in Non-small Cell Lung Cancer. Sci. Rep. 2016, 6, 29719.
- Wang, X.; Sun, Q.; Liu, Q.; Wang, C.; Yao, R.; Wang, Y. CTC immune escape mediated by PD-L1. Med. Hypotheses 2016, 93, 138–139.
- Twomey, J.; Zhang, B. Circulating Tumor Cells Develop Resistance to TRAIL-Induced Apoptosis Through Autophagic Removal of Death Receptor 5: Evidence from an In Vitro Model. Cancers 2019, 11, 94.
- Han, H.; Sung, J.Y.; Kim, S.-H.; Yun, U.-J.; Kim, H.; Jang, E.-J.; Yoo, H.-E.; Hong, E.K.; Goh, S.-H.; Moon, A.; et al. Fibronectin regulates anoikis resistance via cell aggregate formation. Cancer Lett. 2021, 508, 59–72.
- Mitchell, M.J.; King, M.R. Fluid shear stress sensitizes cancer cells to receptor-mediated apoptosis via trimeric death receptors. New J. Phys. 2013, 15, 015008.
- Ward, M.P.; Kane, E.L.; Norris, A.L.; Mohamed, B.M.; Kelly, T.; Bates, M.; Clarke, A.; Brady, N.; Martin, C.M.; Brooks, R.D.; et al. Platelets, immune cells and the coagulation cascade; friend or foe of the circulating tumour cell? Mol. Cancer 2021, 20, 59.
- Liu, X.; Taftaf, R.; Kawaguchi, M.; Chang, Y.-F.; Chen, W.; Entenberg, D.; Zhang, Y.; Gerratana, L.; Huang, S.; Patel, D.B.; et al. Homophilic CD44 Interactions Mediate Tumor Cell Aggregation and Polyclonal Metastasis in Patient-Derived Breast Cancer Models. Cancer Discov. 2019, 9, 96–113.
- Szczerba, B.M.; Castro-Giner, F.; Vetter, M.; Krol, I.; Gkountela, S.; Landin, J.; Scheidmann, M.C.; Donato, C.; Scherrer, R.; Singer, J.; et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 2019, 566, 553–557.
- Hess, K.R.; Varadhachary, G.R.; Taylor, S.H.; Wei, W.; Raber, M.N.; Lenzi, R.; Abbruzzese, J.L. Metastatic patterns in adenocarcinoma. Cancer 2006, 106, 1624–1633.
- Wong, G.L.; Abu Jalboush, S.; Lo, H.-W. Exosomal MicroRNAs and Organotropism in Breast Cancer Metastasis. Cancers 2020, 12, 1827.
- Wu, Q.; Li, J.; Zhu, S.; Wu, J.; Chen, C.; Liu, Q.; Wei, W.; Zhang, Y.; Sun, S. Breast cancer subtypes predict the preferential site of distant metastases: A SEER based study. Oncotarget 2017, 8, 27990–27996.
- Yu, T.; Wang, C.; Xie, M.; Zhu, C.; Shu, Y.; Tang, J.; Guan, X. Heterogeneity of CTC contributes to the organotropism of breast cancer. Biomed. Pharmacother. 2021, 137, 111314.
- Padua, D.; Zhang, X.H.-F.; Wang, Q.; Nadal, C.; Gerald, W.L.; Gomis, R.R.; Massagué, J. TGFβ Primes Breast Tumors for Lung Metastasis Seeding through Angiopoietin-like 4. Cell 2008, 133, 66–77.
- Zhang, X.H.-F.; Wang, Q.; Gerald, W.; Hudis, C.A.; Norton, L.; Smid, M.; Foekens, J.A.; Massagué, J. Latent Bone Metastasis in Breast Cancer Tied to Src-Dependent Survival Signals. Cancer Cell 2009, 16, 67–78.
- Yoneda, T.; Williams, P.J.; Hiraga, T.; Niewolna, M.; Nishimura, R. A Bone-Seeking Clone Exhibits Different Biological Properties from the MDA-MB-231 Parental Human Breast Cancer Cells and a Brain-Seeking Clone In Vivo and In Vitro. J. Bone Miner. Res. 2001, 16, 1486–1495.
- Hoshino, A.; Costa-Silva, B.; Shen, T.-L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335.
- Osmani, N.; Follain, G.; García León, M.J.; Lefebvre, O.; Busnelli, I.; Larnicol, A.; Harlepp, S.; Goetz, J.G. Metastatic Tumor Cells Exploit Their Adhesion Repertoire to Counteract Shear Forces during Intravascular Arrest. Cell Rep. 2019, 28, 2491–2500.
- Yadavalli, S.; Jayaram, S.; Manda, S.S.; Madugundu, A.K.; Nayakanti, D.S.; Tan, T.Z.; Bhat, R.; Rangarajan, A.; Chatterjee, A.; Gowda, H.; et al. Data-Driven Discovery of Extravasation Pathway in Circulating Tumor Cells. Sci. Rep. 2017, 7, 43710.
- Sang A Park and Young-Min Hyuncorresponding author Neutrophil Extravasation Cascade: What Can We Learn from Two-photon Intravital Imaging? Immune Netw. 2016, 16, 317–321.
- Yang, J.; Nie, J.; Ma, X.; Wei, Y.; Peng, Y.; Wei, X. Targeting PI3K in cancer: Mechanisms and advances in clinical trials. Mol. Cancer 2019, 18, 26.
- Zhang, X.H.-F.; Jin, X.; Malladi, S.; Zou, Y.; Wen, Y.H.; Brogi, E.; Smid, M.; Foekens, J.A.; Massagué, J. Selection of Bone Metastasis Seeds by Mesenchymal Signals in the Primary Tumor Stroma. Cell 2013, 154, 1060–1073.
- Hosaka, K.; Yang, Y.; Seki, T.; Fischer, C.; Dubey, O.; Fredlund, E.; Hartman, J.; Religa, P.; Morikawa, H.; Ishii, Y.; et al. Pericyte–fibroblast transition promotes tumor growth and metastasis. Proc. Natl. Acad. Sci. USA 2016, 113, E5618–E5627.
- Lugassy, C.; Kleinman, H.; Barnhill, R. Pericyte mimicry: An embryogenesis-derived program of extravascular tumor cell migration. In Tumor Vascularization; Elsevier: Amsterdam, The Netherlands, 2020; pp. 49–88. ISBN 978-0-12-819494-2.
- Hurtado, P.; Martínez-Pena, I.; Piñeiro, R. Dangerous Liaisons: Circulating Tumor Cells (CTCs) and Cancer-Associated Fibroblasts (CAFs). Cancers 2020, 12, 2861.
- Zeng, Q.; Li, W.; Lu, D.; Wu, Z.; Duan, H.; Luo, Y.; Feng, J.; Yang, D.; Fu, L.; Yan, X. CD146, an epithelial-mesenchymal transition inducer, is associated with triple-negative breast cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 1127–1132.
- Mostert, B.; Kraan, J.; Bolt-de Vries, J.; van der Spoel, P.; Sieuwerts, A.M.; Schutte, M.; Timmermans, A.M.; Foekens, R.; Martens, J.W.M.; Gratama, J.-W.; et al. Detection of circulating tumor cells in breast cancer may improve through enrichment with anti-CD146. Breast Cancer Res. Treat. 2011, 127, 33–41.
- Imbert, A.-M.; Garulli, C.; Choquet, E.; Koubi, M.; Aurrand-Lions, M.; Chabannon, C. CD146 Expression in Human Breast Cancer Cell Lines Induces Phenotypic and Functional Changes Observed in Epithelial to Mesenchymal Transition. PLoS ONE 2012, 7, e43752.
- Mayo, V.; Bowles, A.; Wubker, L.; Ortiz, I.; Cordoves, A.; Cote, R.; Correa, D.; Agarwal, A. Human-derived osteoblast-like cells and pericyte-like cells induce distinct metastatic phenotypes in primary breast cancer cells. Exp. Biol. Med. 2021, 246, 971–985.
- Zhang, D.; LaFortune, T.A.; Krishnamurthy, S.; Esteva, F.J.; Cristofanilli, M.; Liu, P.; Lucci, A.; Singh, B.; Hung, M.-C.; Hortobagyi, G.N.; et al. Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Reverses Mesenchymal to Epithelial Phenotype and Inhibits Metastasis in Inflammatory Breast Cancer. Clin. Cancer Res. 2009, 15, 6639–6648.
- Huang, P.; Chen, A.; He, W.; Li, Z.; Zhang, G.; Liu, Z.; Liu, G.; Liu, X.; He, S.; Xiao, G.; et al. BMP-2 induces EMT and breast cancer stemness through Rb and CD44. Cell Death Discov. 2017, 3, 17039.
- Armbrecht, L.; Rutschmann, O.; Szczerba, B.M.; Nikoloff, J.; Aceto, N.; Dittrich, P.S. Quantification of Protein Secretion from Circulating Tumor Cells in Microfluidic Chambers. Adv. Sci. 2020, 7, 1903237.
- Park, J.; Park, S.; Hyun, K.A.; Jung, H.-I. Microfluidic recapitulation of circulating tumor cell–neutrophil clusters via double spiral channel-induced deterministic encapsulation. Lab. Chip 2021, 21, 3483–3497.
- Wheeler, S.E.; Clark, A.M.; Taylor, D.P.; Young, C.L.; Pillai, V.C.; Stolz, D.B.; Venkataramanan, R.; Lauffenburger, D.; Griffith, L.; Wells, A. Spontaneous dormancy of metastatic breast cancer cells in an all human liver microphysiologic system. Br. J. Cancer 2014, 111, 2342–2350.
- Riahi, R.; Yang, Y.L.; Kim, H.; Jiang, L.; Wong, P.K.; Zohar, Y. A microfluidic model for organ-specific extravasation of circulating tumor cells. Biomicrofluidics 2014, 8, 024103.
- Aleman, J.; Skardal, A. A multi-site metastasis-on-a-chip microphysiological system for assessing metastatic preference of cancer cells: ALEMAN AND SKARDAL. Biotechnol. Bioeng. 2019, 116, 936–944.
- Song, J.; Miermont, A.; Lim, C.T.; Kamm, R.D. A 3D microvascular network model to study the impact of hypoxia on the extravasation potential of breast cell lines. Sci. Rep. 2018, 8, 17949.
- Marturano-Kruik, A.; Nava, M.M.; Yeager, K.; Chramiec, A.; Hao, L.; Robinson, S.; Guo, E.; Raimondi, M.T.; Vunjak-Novakovic, G. Human bone perivascular niche-on-a-chip for studying metastatic colonization. Proc. Natl. Acad. Sci. USA 2018, 115, 1256–1261.
- Jeon, J.S.; Bersini, S.; Gilardi, M.; Dubini, G.; Charest, J.L.; Moretti, M.; Kamm, R.D. Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation. Proc. Natl. Acad. Sci. USA 2015, 112, 214–219.
- Bersini, S.; Jeon, J.S.; Dubini, G.; Arrigoni, C.; Chung, S.; Charest, J.L.; Moretti, M.; Kamm, R.D. A microfluidic 3D in vitro model for specificity of breast cancer metastasis to bone. Biomaterials 2014, 35, 2454–2461.
- Mei, X.; Middleton, K.; Shim, D.; Wan, Q.; Xu, L.; Ma, Y.-H.V.; Devadas, D.; Walji, N.; Wang, L.; Young, E.W.K.; et al. Microfluidic platform for studying osteocyte mechanoregulation of breast cancer bone metastasis. Integr. Biol. 2019, 11, 119–129.
- Xu, H.; Li, Z.; Yu, Y.; Sizdahkhani, S.; Ho, W.S.; Yin, F.; Wang, L.; Zhu, G.; Zhang, M.; Jiang, L.; et al. A dynamic in vivo-like organotypic blood-brain barrier model to probe metastatic brain tumors. Sci. Rep. 2016, 6, 36670.
This entry is offline, you can click here to edit this entry!
