Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biology
Adrenomedullin (AM) and proadrenomedullin N-terminal 20 peptide (PAMP) are two bioactive peptides derived from the same precursor with several biological functions including vasodilation, angiogenesis, or anti-inflammation, among others. AM and PAMP are widely expressed throughout the gastrointestinal (GI) tract where they behave as GI hormones, regulating numerous physiological processes such as gastric emptying, gastric acid release, insulin secretion, bowel movements, or intestinal barrier function.
- adrenomedullin
- PAMP
- microbiota
- gastrointestinal peptides
- intestinal physiology
- intestinal pathology
- inflammatory bowel disease
1. Introduction
The digestive system is composed of the oral cavity, salivary glands, gastrointestinal (GI) tract, the liver, and the exocrine pancreas. The principal functions of the GI tract are to digest and absorb ingested nutrients and to excrete waste products of digestion [1]; but they are not limited to those. The GI tract is the key interface between ingested nutrients and the body, and it plays a critical role in regulating energy homeostasis [2]. Furthermore, recent findings have demonstrated that the events that take place in the gut during early life contribute to the programming, shaping, and tuning of GI tract physiology, the microbiome, and the immune system, having lifelong health consequences [3].
The GI tract can control and exert different actions in other organs thanks to multiple GI-derived signals including peptides/hormones, bile acids, and biomolecules [4]. In fact, the GI tract represents the largest endocrine organ in the body thanks to enteroendocrine cells (EECs) harbored all along the gut. EECs are the primary sensors of changes that take place in the GI tract, and they synthesize and release an array of peptides and hormones that act as autocrine, paracrine, or endocrine regulators of digestive function, glucose homeostasis, and energy balance, among other functions [5]. Over 100 different peptides with hormonal activity are produced and released by EECs as well as neurons distributed along the GI tract. GI peptides/hormones play an integral role in regulating the functions of the GI tract. In addition to their regulatory effects on secretion, absorption, digestion, and gut motility, they also have a fundamental role in gut–brain communication, particularly in the control of food intake and energy homeostasis [6]. Several GI-derived peptides are also synthesized within the brain, where they act as neuromodulators and/or neurotransmitters [4]. In this context, another important player has more recently emerged, the gut microbiota, whose interaction with GI hormones seems to have a key role in the effects of diets and improving the treatment of some intestinal disorders [7][8]. This adds a layer of complexity in terms of characterizing the physiologic role of GI-derived peptides.
Adrenomedullin (AM) and proadrenomedullin N-terminal 20 peptide (PAMP) are two small active hormones derived from the expression of a single gene (Adm) that is expressed throughout the GI tract, including the mucosal epithelium, glandular duct cells, neuroendocrine cells, and smooth muscle cells of the GI tract, between the oral cavity and the rectum [9][10][11][12]. These two peptides coexist in GI cells, where they regulate many physiological functions including vasodilation, angiogenesis, anti-inflammation, organ protection, and tissue repair. AM suppresses inflammatory cytokine production in the intestinal mucosa, improves vascular and lymphatic function, mucosal epithelial repair, and intestinal barrier function in animal models with intestinal inflammation [10][13][14][15]. Different research groups have demonstrated that AM has a protective role in many GI diseases, and its administration in rodents and humans ameliorates the severity of different gut pathologies such as gastric ulcer [16] or inflammatory bowel disease (IBD) [17][18]. Since AM is an endogenous bioactive peptide, it has low immunogenicity and is considered relatively safe, so it is expected that novel AM- and/or PAMP-derived treatments for GI pathologies may be developed in the coming years [19].
2. Adrenomedullin and Proadrenomedullin N-Terminal 20 Peptide
AM was initially discovered in 1993 by Kitamura et al. [20] when it was isolated from a human pheochromocytoma. AM was initially studied as a peptide able to activate platelet adenylate cyclase and exert a long-lasting hypotensive effect [20], but it was later found to promote angiogenesis, organ protection, and anti-inflammatory immune activity [9].
2.1. Adrenomedullin and PAMP Biosynthesis and Structure
AM and PAMP are both coded by the Adm gene located in human chromosome 11p15.4 and in mouse chromosome 7. This gene contains four exons and three introns, with TATA, CAAT, and GC boxes in the 5’-flanking region [21]. The Adm gene codes for a large precursor, preproAM, of 185 amino acids. PreproAM is converted into proAM after the cleaving of the 21-residue signaling peptide. The enzymatic processing of proAM generates both mature AM (amino acids 95–146 of preproAM) and PAMP (amino acids 22–41 of preproAM) [22]. An interesting fact is that although both AM and PAMP are produced by the same prohormone precursor, their expression may not coincide in the same cells due to the existence of an alternative splicing mechanism [23].
Human AM is composed of 52 amino acids and has a ring structure consisting of six amino acids and a C-terminal amide structure. Both structural features are essential for its biological activity. AM shares structural similarities with calcitonin gene-related peptide (CGRP), amylin, and intermedin (also known as AM2) [22][24][25]. Another common structural characteristic of the members of the CGRP family is the presence of a central alpha helix. In the case of AM, approximately one-third of its total length is occupied by this central helical region, which seems to be required for binding to specific receptors and, thus, for exerting physiological functions [26]. PAMP is also constituted by an alpha helix, which is important not only for receptor recognition but also for some of its actions such as its antimicrobial activity [27][28].
AM is widely expressed throughout the blood vessels, heart, lungs, kidneys, central nervous system, and GI tract, among others, and is highly concentrated in the adrenal medulla. PAMP has a shorter antihypertensive activity than AM and cooperatively regulates blood circulation with AM [23].
2.2. Adrenomedullin and PAMP Receptors
The AM receptor consists of a complex of a seven transmembrane domain protein (calcitonin receptor-like receptor (CLR)) and a single transmembrane domain protein (receptor activity modifying protein (RAMP)). After one of the RAMPs bind to CLR in the endoplasmatic reticulum, the resulting complex is transported to the plasma membrane [30].
There are three different RAMP isoforms in the human genome: RAMP1, RAMP2, and RAMP3. The complex formed by the union of CLR and RAMP1 functions as a receptor for the CGRP peptide [30]. The CLR molecules that bind to RAMP2 or RAMP3 isoforms are core-glycosylated, and these are the complexes that work as AM receptors (AMR). CLR/RAMP2 is known as AMR1, whereas CLR/RAMP3 is called AMR2 [31]. It has been established that amino acid 74 in RAMP2 and RAMP3 is critical for their affinity for AM, while amino acid 93 in RAMP1 is mainly responsible for its affinity for CGRP [31].
RAMP2 is more abundantly expressed under physiological conditions. However, the balance between the expression of RAMP2 and RAMP3 in a particular cell type can change, determining the degree of response to AM [32]. Apparently, the elevation in RAMP3 expression occurs as a mechanism to decrease AM’s responsiveness in those situations in which AM levels are most elevated such as in pregnancy, sepsis, or heart failure [32].
Specific binding sites for AM have been described in almost all tissues and cell types, including heart, lung, liver, spleen, skeletal muscle, kidney, GI tract, brain, or spinal cord, among others [12], providing the anatomical basis for the involvement of AM in the physiology of all those organs.
PAMP differs in size and sequence from AM; thus, it has no affinity to the AMR complex CLR/RAMPs. Instead, the Mas-related G protein-coupled receptor member X2 was proposed as the receptor for PAMP as well as for its endogenously processed form, PAMP-12 (consisting of amino acids 9–20 of the PAMP’s mature form) [33]. Some publications have shown that the cytoskeleton can also function as a sort of intracellular receptor of PAMP (34). Physiological experiments show that PAMP contributes to microtubule fluidity and increases kinesin speed [34].
Recent studies have proposed that atypical chemokine receptors (ACKRs) can also act as AM/PAMP receptors [35]. ACKRs are vital regulators of the spatiotemporal distribution of chemokines [36]. ACKR3, formerly named CXCR7, is expressed ubiquitously but is most abundantly present in different brain regions, adrenal glands, lymphatic and blood vasculature, heart, and various subsets of immune cells [37]. AM seems to be the only member of the CGRP peptide superfamily that moderately activates ACKR3 [35]. Remarkably, PAMP has an activity toward ACKR3 that is comparable to AM. Furthermore, its truncated analog, PAMP-12, shows a greater potency toward ACKR3 than AM [35].
2.3. Main Physiological Effects of Adrenomedullin and PAMP
AM/PAMP play a main role during mammalian embryonic development. Both peptides can be detected in the uterus, the placenta, and several fetal tissues [38]. Furthermore, AM is locally produced in the trophoblast binucleate cells of the bovine placenta, where it may play a critical role regulating placenta vascularization during pregnancy, especially during the transition of late gestational period [39]. The generation of different knockout (KO) models for the Adm gene support the idea that AM is intimately related with pregnancy and embryonic development. In KO mouse models where AM and PAMP synthesis are suppressed, the null phenotype is embryonically lethal [40][41]. The same results were observed when AM receptors are suppressed; CLR−/− and RAMP2−/− embryos die in utero at mid-gestation due to severe deformation, vascular fragility, severe edema, and hemorrhage [42][43]. Surprisingly, no survival problems were observed when the expression of RAMP3 was totally suppressed [44].
To circumvent the problem of embryo lethality, tissue-specific conditional KO models were generated to study the actions exerted by AM/PAMP in adult organisms [45].
Systemic administration of AM reduces arterial blood pressure, decreases peripheral vascular resistance, and increases heart rate and cardiac output [46]. Although AM acts as a vasodilator when administered peripherally, it acts as a vasoconstrictor when it is injected into the brain, probably acting through vascular nerve terminals [47]. AM and PAMP are also well known for being potent angiogenic peptides [48], they are necessary for maintaining the integrity of the microvasculature [49] and promoting a faster healing of epithelial wounds [50][51].
Acting directly in the kidneys and through the hypothalamic–pituitary axis, AM is able to control renal function and regulate body fluid volume [52][53]. Interestingly, PAMP is expressed in the juxtaglomerular complex and co-secreted with renin, so this small peptide may be also involved in fluid volume control [54].
AM also regulates the secretion of other hormones. Maybe the best described to date is the regulatory effect of AM on blood glycemic levels via regulating insulin secretion. AM has been shown to reduce insulin secretion, and the use of a blocking monoclonal antibody against AM was able to increase the insulin secretion rate five-fold [55]. AM also regulates ghrelin secretion in the mouse stomach through an indirect mechanism mediated by plasmatic glucose levels [56], as it has been shown that low glucose induces ghrelin secretion from the stomach [57].
AM and its receptors are widely and abundantly expressed in the central nervous system [58]. This small hormone is an important neuroprotective agent against ischemic damage [59], increases preganglionic sympathetic discharges [60], and regulates some properties of the blood–brain barrier [61]. In addition, it has been described that AM may be able to regulate some behavioral responses such as stress and nociception [62][63].
Recently, a total inducible KO model for AM using Cre/LoxP technology combined with an optimized form of reverse tetracycline-controlled transactivator has been generated [56]. This new model has allowed the deep characterization of some of the already described actions for AM, and has led to the discovery of new ones, including its activity in bone remodeling [56] or the regulation of gut microbiota [14.
3. Adrenomedullin/PAMP’s Roles in the Digestive System under Physiological Conditions
AM and PAMP are widely expressed throughout the mucosal epithelium, glandular duct cells, neuroendocrine cells, and smooth muscle cells of the GI tract, between the oral cavity and the rectum [10] (Figure 1). The wide distribution of AM and PAMP in the GI tract suggests that both peptides regulate many digestive functions under physiological conditions.
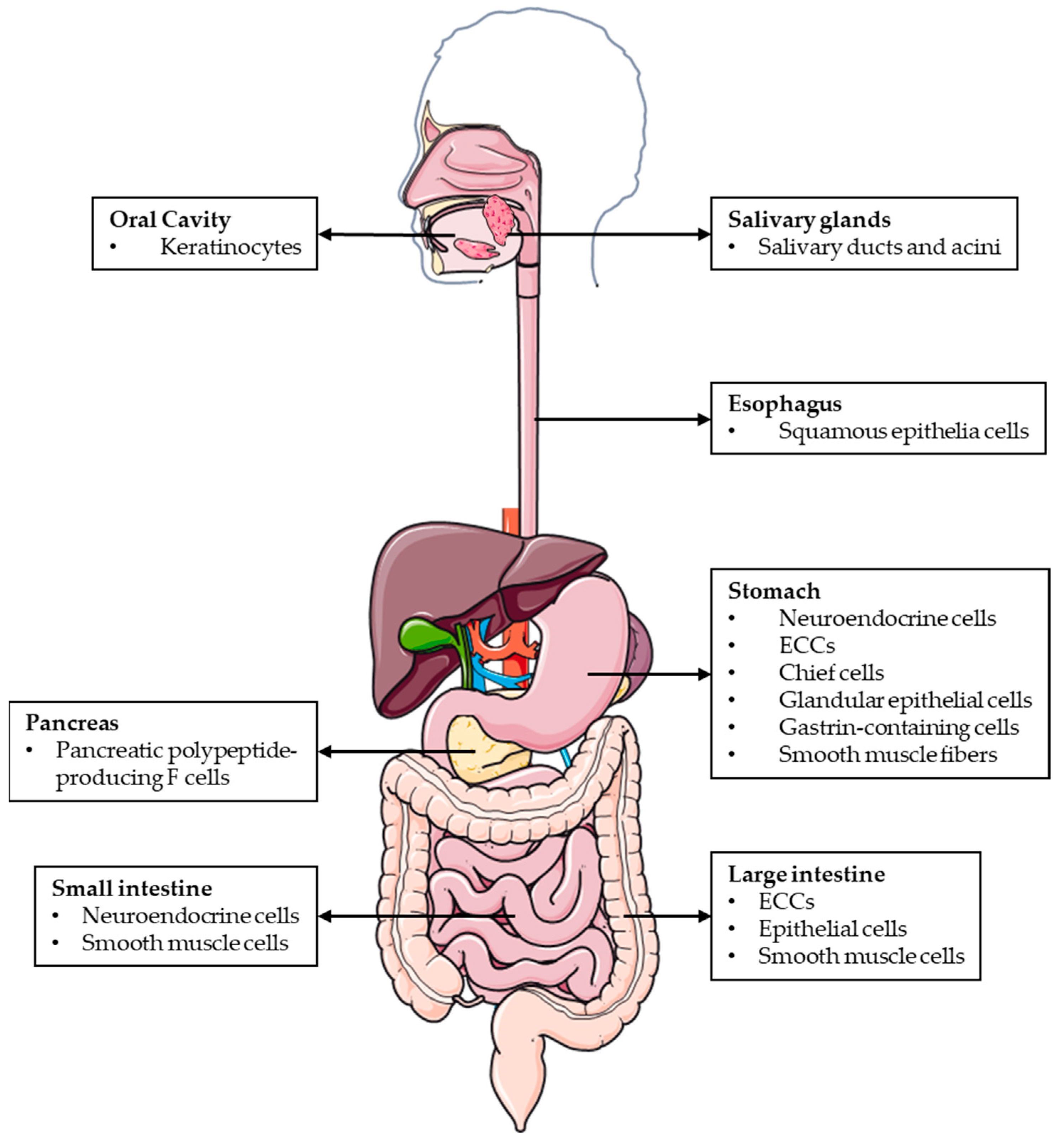
Figure 1. Schematic representation of the different locations where AM and PAMP are expressed throughout the GI tract.
3.1. Adrenomedullin Actions in the Oral Cavity
AM can be detected in the saliva, and it is secreted from oral keratinocytes and salivary glands [11][64]. Salivary AM seems to increase oral keratinocyte growth and to inhibit specific bacterial growth in the mouth in a dose-dependent manner [64]. Human oral keratinocytes resist bacterial infection, in part, by producing a broad-spectrum of antimicrobial peptides, including AM, to provide a robust response against pathogens [65]. Furthermore, microarray-profiling studies of dental caries revealed that the Adm gene is upregulated in pulp disease [66], probably as a defensive mechanism.
3.2. Role of Adrenomedullin in Stomach Physiology
The expression sites of AM and its receptors in the stomach (especially abundant in the ECCs and chief cells of the gastric fundus and in the base of the glandular epithelia in the pyloric mucosa [67][68][69]) suggest that AM may play a role in regulating gastric functions in a paracrine manner.
AM was first described as a potent inhibitor of basal gastric acid secretion in 1997 [70]. Further studies confirmed that AM was extremely potent in inhibiting basal and bombesin-, histamine-, and 2-deoxy D-glucose-stimulated gastric acid secretion [71]. Further, ex vivo studies suggested that AM acts via intramural fundic neurons stimulating somatostatin and, thus, inhibiting histamine and acid secretion in the stomach [72].
3.3. Adrenomedullin and PAMP Actions in the Intestine
AM expression in the intestine seems to be species specific. In the rat, half of the AM-like immunoreactivity is detected in the colon [69][78], while the ileum expresses the highest levels of preproAM among the small intestine [79]. AM immunostained cells can be localized in all intestine layers, intestinal nerves [80], and smooth muscle cells [81]. However, the major form of AM immunoreactivity in the colon corresponds with ECCs [78]. In the porcine GI tract, duodenal AM levels are about four to fourteen times higher than in other GI tissues, being more abundant in the mucosa and submucosa [82]. In human colonic mucosa, AM and PAMP immunostained cells are observed at a higher concentration in the apical region of the epithelium [11].
The fact that GI visceral smooth muscle cells express AM [69] suggests that AM may be able to regulate the contractile responses in the gut. This was proven in 2004, when AM elicited relaxation of the rat ileum in a concentration-dependent manner, acting through β3-adrenoreceptors [83]. Furthermore, intravenously injected AM disrupts phase 3 of the migrating motor complex (the cyclic motility pattern exhibited by the small intestine during fasting) in rat jejunum [79]. The migrating motor complex is regulated by a complex neuronal mechanism [84]; thus, AM control of the small-intestinal motility is probably achieved by acting as a neurotransmitter. Moreover, AM is able to regulate colonic bowel movements [81], causing a dose-dependent persistent relaxation of the rat colonic smooth muscle by elevating cAMP levels [81].
AM and PAMP can regulate sugar absorption by the enterocytes [85]. PAMP enhances sugar uptake, while AM inhibits the absorption of α-methylglucoside. Both peptides regulate sugar absorption via the recruitment of the sodium glucose co-transporter-1 (SGLT1) to the apical membrane [85]. AM also actively regulates water and ion absorption and transport in the colon [81]. This has two important effects on ion transport: it decreases sodium absorption and enhances chloride secretion [81]. By altering ion flow, AM also modulates water absorption in the colon.
3.4. Role of Adrenomedullin in Pancreatic Physiology
In the pancreas, AM expression appears early in embryonic development. Specifically, AM immunoreactivity appears at day 11.5 of embryonic development in the rat [86]. At some point during development, all pancreatic cell types express AM, but this evolves towards the adult pattern where AM is only express by the pancreatic polypeptide-producing F cells on the periphery of pancreatic islets of Langerhans [87].
The early appearance of AM during embryonic development suggests an active role in the growth and morphogenesis of the organ [86]. However, the best known action of AM in the pancreas is the inhibition of insulin secretion, thus modulating blood glucose levels [87]. AM inhibits insulin secretion in a dose-dependent manner, increasing circulating glucose levels at the same time, and a monoclonal antibody against AM is able to increase insulin release five-fold [87]. The inhibition of glucose-induced insulin secretion by AM was restored in the pancreatic β cells pretreating the cells with pertussis toxin, suggesting that this effect could be mediated by G proteins [88] and an elevation of cAMP [89].
Recent studies have provided evidence that AM/PAMP are involved in the regulation of ghrelin levels [56]. Ghrelin levels in the blood of total KO mice were significantly higher than in the WT littermates [56]. This may be a consequence of AM regulation of insulin secretion in the pancreas. Animals lacking AM/PAMP have lower fasting basal glucose levels than WT animals, and these differences are maintained through a glucose tolerance test [56]. It has been shown that low glucose induces ghrelin secretion from the stomach [57], so ghrelin regulation by AM/PAMP may be achieved through indirect mechanisms involving glycemic levels.
AM is able to inhibit amylase secretion too, probably acting through a GTP-binding protein and reducing the calcium sensitivity of the exocytotic machinery of the pancreatic acini [90], however, this mechanism is not yet fully understood.
3.5. Adrenomedullin/PAMP’s Impact on Microbiota Composition
Gut microbiota has emerged as a main regulator for many GI functions as well as a major player for maintaining a healthy GI function [91][92]; thus, any hormone/peptide able to regulate microbiota composition may have an impact on GI physiology.
AM and PAMP are found in mostly all epithelial surfaces and body secretions including saliva, sweat, milk, and urine [22][93]; this suggests that both of them may play a role as antimicrobial peptides, contributing to host defense [94]. This was further confirmed by different studies using in vitro, in vivo, or clinical data.
Plasmatic and GI AM levels increase in many infectious diseases [80][94][95], especially in sepsis [96], suggesting a protective role for this hormone.
The antimicrobial activity of AM and PAMP was demonstrated in 1996 [97] for the first time. These two peptides were able to suppress the growth of Gram-positive and Gram-negative bacteria in a concentration- and time-dependent manner [98][99][100].
AM shares many properties with other cationic antimicrobial peptides, including human β-defensin-2, and therefore may share a similar antimicrobial mechanism of action [94]. Ultrastructural studies have demonstrated that AM treatment causes a cell-wall disruption in E. coli and an abnormal septum formation in S. aureus [101]. This antimicrobial activity seems to be related with the carboxy terminal fragment of AM [101].
In the case of PAMP, a conformational analysis revealed that its structure is compatible with a pore-forming mechanism [27]. Antimicrobial analysis using radial diffusion and outer membrane permeability assays showed that free-acid and mature PAMP are very efficient in increasing outer membrane permeability in E. coli, in a similar way to polymyxin B [28] thus confirming the suspected antimicrobial mechanism of action for PAMP.
The inducible total KO model for AM and PAMP has allowed the confirmation of the effects of eliminating the Adm gene on gut microbiota in physiological conditions [14]. Abrogation of the Adm gene in the whole body caused significant changes in colonic microbiota: higher proportion of the Proteobacteria class and the Coriobacteriales order, and other families and genera was observed in KO feces. Meanwhile, these mice had a lower proportion of beneficial bacteria such as Lactobacillus gasseri and Bifidobacterium choerinum [14]. All together, these data point to a beneficial effect of AM/PAMP on GI tract health.
This entry is adapted from the peer-reviewed paper 10.3390/biom12020156
References
- Hajishafiee, M.; Bitarafan, V.; Feinle-Bisset, C. Gastrointestinal Sensing of Meal-Related Signals in Humans, and Dysregulations in Eating-Related Disorders. Nutrients 2019, 11, 1298.
- Monteiro, M.P.; Batterham, R.L. The Importance of the Gastrointestinal Tract in Controlling Food Intake and Regulating Energy Balance. Gastroenterology 2017, 152, 1707–1717.
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Palacio, S.D.; Montes, S.A.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17.
- Dockray, G.J. Gastrointestinal hormones and the dialogue between gut and brain. J. Physiol. 2014, 30, 165–169.
- Guo, X.; Lv, J.; Xi, R. The specification and function of enteroendocrine cells in Drosophila and mammals: A comparative review. FEBS J. 2021. online ahead of print.
- Dockray, G.J. Enteroendocrine cell signalling via the vagus nerve. Curr. Opin. Pharmacol. 2013, 13, 954–958.
- Xu, X.; Chen, R.; Zhan, G.; Wang, D.; Tan, X.; Xu, H. Enterochromaffin Cells: Sentinels to Gut Microbiota in Hyperalgesia? Front. Cell Infect. Microbiol. 2021, 11, 760076.
- Cussotto, S.; Sandhu, K.V.; Dinan, T.G.; Cryan, J.F. The Neuroendocrinology of the Microbiota-Gut-Brain Axis: A Behavioural Perspective. Front. Neuroendocrinol. 2018, 51, 80–101.
- Hinson, J.P.; Kapas, S.; Smith, D.M. Adrenomedullin, a multifunctional regulatory peptide. Endocr. Rev. 2000, 21, 138–167.
- Martinez-Herrero, S.; Martinez, A. Adrenomedullin regulates intestinal physiology and pathophysiology. Domest. Anim. Endocrinol. 2016, 56 (Suppl. S66–S83).
- Marutsuka, K.; Hatakeyama, K.; Sato, Y.; Yamashita, A.; Sumiyoshi, A.; Asada, Y. Immunohistological localization and possible functions of adrenomedullin. Hypertens Res. 2003, 26 (Suppl. S33–S40), S33–S40).
- Lopez, J.; Martinez, A. Cell and molecular biology of the multifunctional peptide, adrenomedullin. Int. Rev. Cytol. 2002, 221, 1–92.
- Martínez-Herrero, S.; Pérez-Matute, P.; Villanueva-Millán, M.; Oteo, J.; Martínez, A. Changes in Gut Microbiota Induced by Lack of Adrenomedullin. Microbiology ASF. In Proceedings of the 54th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC®), Washington, DC, USA, 6 September 2014.
- Martinez-Herrero, S.; Larrayoz, I.M.; Narro-Iniguez, J.; Villanueva-Millan, M.J.; Recio-Fernandez, E.; Perez-Matute, P.; Oteo, J.A.; Martínez, A. Lack of Adrenomedullin Results in Microbiota Changes and Aggravates Azoxymethane and Dextran Sulfate Sodium-Induced Colitis in Mice. Front. Physiol. 2016, 7, 595.
- Martinez-Herrero, S.; Larrayoz, I.M.; Narro-Iniguez, J.; Rubio-Mediavilla, S.; Martinez, A. Lack of Adrenomedullin Aggravates Acute TNBS-Induced Colitis Symptoms in Mice, Especially in Females. Front. Physiol. 2017, 8, 1058.
- Clementi, G.; Caruso, A.; Cutuli, V.M.; de Bernardis, E.; Prato, A.; Mangano, N.G.; Amico-Roxas, M. Effects of centrally of peripherally injected adrenomedullin on reserpine-induced gastric lesions. Eur. J. Pharmacol. 1998, 36, 51–54.
- Ashizuka, S.; Inatsu, H.; Kita, T.; Kitamura, K. Adrenomedullin Therapy in Patients with Refractory Ulcerative Colitis: A Case Series. Dig. Dis. Sci. 2016, 61, 872–880.
- Ashizuka, S.; Kuroishi, N.; Nakashima, K.; Inatsu, H.; Kita, T.; Kitamura, K. Adrenomedullin: A Novel Therapy for Intractable Crohn’s Disease with a Loss of Response to Infliximab. Intern. Med. 2019, 58, 1573–1576.
- Ashizuka, S.; Kita, T.; Inatsu, H.; Kitamura, K. Adrenomedullin: A Novel Therapeutic for the Treatment of Inflammatory Bowel Disease. Biomedicines 2021, 9, 1068.
- Kitamura, K.; Kangawa, K.; Kawamoto, M.; Ichiki, Y.; Nakamura, S.; Matsuo, H.; Eto, T. Adrenomedullin: A novel hypotensive peptide isolated from human pheochromocytoma. Biochem. Biophys. Res. Commun. 1993, 192, 553–560.
- Ishimitsu, T.; Kojima, M.; Kangawa, K.; Hino, J.; Matsuoka, H.; Kitamura, K.; Eto, T.; Matsuo, H. Genomic structure of human adrenomedullin gene. Biochem. Biophys. Res. Commun. 1994, 203, 631–639.
- Beltowski, J.; Jamroz, A. Adrenomedullin—What do we know 10 years since its discovery? Pol. J. Pharmacol. 2004, 56, 5–27.
- Martínez, A.; Hodge, D.L.; Garayoa, M.; Young, H.A.; Cuttitta, F. Alternative splicing of the proadrenomedullin gene results in differential expression of gene products. J. Mol. Endocrinol. 2001, 27, 31–41.
- Takei, Y.; Inoue, K.; Ogoshi, M.; Kawahara, T.; Bannai, H.; Miyano, S. Identification of novel adrenomedullin in mammals: A potent cardiovascular and renal regulator. FEBS Lett. 2004, 556, 53–58.
- Roh, J.; Chang, C.L.; Bhalla, A.; Klein, C.; Hsu, S.Y. Intermedin is a calcitonin/calcitonin gene-related peptide family peptide acting through the calcitonin receptor-like receptor/receptor activity-modifying protein receptor complexes. J. Biol. Chem. 2004, 279, 7264–7274.
- Perez-Castells, J.; Martin-Santamaria, S.; Nieto, L.; Ramos, A.; Martinez, A.; de Pascual-Teresa, B.; Jimenez-Barbero, J. Structure of Micelle-Bound Adrenomedullin: A First Step Toward the Analysis of Its Interactions with Receptors and Small Molecules. Biopolymers 2012, 97, 45–53.
- Lucyk, S.; Taha, H.; Yamamoto, H.; Miskolzie, M.; Kotovych, G. NMR conformational analysis of proadrenomedullin N-terminal 20 peptide, a proangiogenic factor involved in tumor growth. Biopolymers 2006, 81, 295–308.
- Martinez, A.; Bengoechea, J.A.; Cuttitta, F. Molecular evolution of proadrenomedullin N-terminal 20 peptide (PAMP): Evidence for gene co-option. Endocrinology 2006, 147, 3457–3461.
- Garayoa, M.; Martínez, A.; Lee, S.; Pío, R.; An, W.G.; Neckers, L.; Trepel, J.; Montuenga, L.M.; Ryan, H.; Johnson, R.; et al. Hypoxia-inducible factor-1 (HIF-1) up-regulates adrenomedullin expression in human tumor cell lines during oxygen deprivation: A possible promotion mechanism of carcinogenesis. Mol. Endocrinol. 2000, 148, 48–62.
- Poyner, D.R.; Sexton, P.M.; Marshall, I.; Smith, D.M.; Quirion, R.; Born, W.; Muff, R.; Fischer, J.A.; Foord, S.M. International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol. Rev. 2002, 54, 233–246.
- Qi, T.; Christopoulos, G.; Bailey, R.J.; Christopoulos, A.; Sexton, P.M.; Hay, D.L. Identification of N-terminal receptor activity-modifying protein residues important for calcitonin gene-related peptide, adrenomedullin, and amylin receptor function. Mol. Pharmacol. 2008, 74, 1059–1071.
- Gibbons, C.; Dackor, R.; Dunworth, W.; Fritz-Six, K.; Caron, K.M. Receptor activity-modifying proteins: RAMPing up adrenomedullin signaling. Mol. Endocrinol. 2007, 21, 783–796.
- Kamohara, M.; Matsuo, A.; Takasaki, J.; Kohda, M.; Matsumoto, M.; Matsumoto, S.; Soga, T.; Hiyama, H.; Katou, M. Identification of MrgX2 as a human G-protein-coupled receptor for proadrenomedullin N-terminal peptides. Biochem. Biophys. Res. Commun. 2005, 330, 1146–1152.
- Larrayoz, I.M.; Martinez-Herrero, S.; Ochoa-Callejero, L.; Garcia-Sanmartin, J.; Martinez, A. Is the Cytoskeleton an Intracellular Receptor for Adrenomedullin and PAMP? Curr. Protein Pept. Sci. 2013, 14, 429–443.
- Meyrath, M.; Palmer, C.B.; Reynders, N.; Vanderplasschen, A.; Ollert, M.; Bouvier, M.; Szpakowska, M.; Chevigné, A. Proadrenomedullin N-Terminal 20 Peptides (PAMPs) Are Agonists of the Chemokine Scavenger Receptor ACKR3/CXCR7. ACS Pharmacol. Transl. Sci. 2021, 48, 13–23.
- Bachelerie, F.; Ben-Baruch, A.; Burkhardt, A.M.; Combadiere, C.; Farber, J.M.; Graham, G.J.; Horuk, R.; Sparre-Ulrich, A.H.; Locati, M.; Luster, A.D.; et al. International Union of Basic and Clinical Pharmacology. . LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol. Rev. 2014, 66, 1–79.
- Regard, J.B.; Sato, I.T.; Coughlin, S.R. Anatomical profiling of G protein-coupled receptor expression. Cell 2008, 135, 561–571.
- Garayoa, M.; Bodegas, E.; Cuttitta, F.; Montuenga, L.M. Adrenomedullin in mammalian embryogenesis. Microsc. Res. Tech. 2002, 57, 40–54.
- Hayashi, K.G.; Hosoe, M.; Sakumoto, R.; Takahashi, T. Temporo-spatial expression of adrenomedullin and its receptors in the bovine placenta. Reprod. Biol. Endocrinol. 2013, 11, 62.
- Shindo, T.; Kurihara, Y.; Nishimatsu, H.; Moriyama, N.; Kakoki, M.; Wang, Y.; Imai, Y.; Ebihara, A.; Kuwaki, T.; Ju, K.-H.; et al. Vascular abnormalities and elevated blood pressure in mice lacking adrenomedullin gene. Circulation 2001, 104, 1964–1971.
- Shimosawa, T.; Shibagaki, Y.; Ishibashi, K.; Kitamura, K.; Kangawa, K.; Kato, S.; Ando, K.; Fujita, T. Adrenomedullin, an endogenous peptide, counteracts cardiovascular damage. Circulation 2002, 105, 106–111.
- Dackor, R.T.; Fritz-Six, K.; Dunworth, W.P.; Gibbons, C.L.; Smithies, O.; Caron, K.M. Hydrops fetalis, cardiovascular defects, and embryonic lethality in mice lacking the calcitonin receptor-like receptor gene. Mol. Cell Biol. 2006, 26, 2511–2518.
- Ichikawa-Shindo, Y.; Sakurai, T.; Kamiyoshi, A.; Kawate, H.; Iinuma, N.; Yoshizawa, T.; Koyama, T.; Fukuchi, J.; Iimuro, S.; Moriyama, N.; et al. The GPCR modulator protein RAMP2 is essential for angiogenesis and vascular integrity. J. Clin. Investig. 2008, 118, 29–39.
- Dackor, R.; Fritz-Six, K.; Smithies, O.; Caron, K. Receptor activity-modifying proteins 2 and 3 have distinct physiological functions from embryogenesis to old age. J. Biol. Chem. 2007, 282, 18094–18099.
- Plück, A. Conditional mutagenesis in mice: The Cre/loxP recombination system. Int. J. Exp. Pathol. 1996, 77, 269–278.
- Nicholls, M.G. Hemodynamic and hormonal actions of adrenomedullin. Braz. J. Med. Biol. Res. 2004, 37, 1247–1253.
- Samson, W.K.; Murphy, T.C.; Resch, Z.T. Central mechanisms for the hypertensive effects of preproadrenomedullin-derived peptides in conscious rats. Am. J. Physiol. 1998, 274, R1505–R1509.
- Martinez, A. A new family of angiogenic factors. Cancer Lett. 2006, 236, 157–163.
- Koyama, T.; Ochoa-Callejero, L.; Sakurai, T.; Kamiyoshi, A.; Ichikawa-Shindo, Y.; Iinuma, N.; Arai, T.; Yoshizawa, T.; Iesato, Y.; Lei, Y.; et al. Vascular Endothelial Adrenomedullin-RAMP2 System Is Essential for Vascular Integrity and Organ Homeostasis. Circulation 2013, 127, 842–853.
- Garcia-Honduvilla, N.; Cifuentes, A.; Bellon, J.M.; Bujan, J.; Martinez, A. The angiogenesis promoter, proadrenomedullin N-terminal 20 peptide (PAMP), improves healing in both normoxic and ischemic wounds either alone or in combination with autologous stem/progenitor cells. Histol. Histopathol. 2013, 28, 115–125.
- Harada, K.; Yamahara, K.; Ohnishi, S.; Otani, K.; Kanoh, H.; Ishibashi-Ueda, H.; Minamino, N.; Kangawa, K.; Nagaya, N.; Ikeda, T. Sustained-release adrenomedullin ointment accelerates wound healing of pressure ulcers. Regul. Pept. 2011, 168, 21–26.
- Nishikimi, T. Adrenomedullin in the kidney-renal physiological and pathophysiological roles. Curr. Med. Chem. 2007, 14, 1689–1699.
- Jougasaki, M.; Wei, C.M.; Aarhus, L.L.; Heublein, D.M.; Sandberg, S.M.; Burnett, J.C. Renal localization and actions of adrenomedullin: A natriuretic peptide. Am. J. Physiol. 1995, 268, 657–663.
- López, J.; Cuesta, N.; Martínez, A.; Montuenga, L.; Cuttitta, F. Proadrenomedullin N-terminal 20 peptide (PAMP) immunoreactivity in vertebrate juxtaglomerular granular cells identified by both light and electron microscopy. Gen. Comp. Endocrinol. 1999, 116, 192–203.
- Zudaire, E.; Cuttitta, F.; Martínez, A. Regulation of pancreatic physiology by adrenomedullin and its binding protein. Regul. Pept. 2003, 112, 121–130.
- Martinez-Herrero, S.; Larrayoz, I.M.; Ochoa-Callejero, L.; Fernandez, L.J.; Allueva, A.; Ochoa, I.; Martínez, A. Prevention of Bone Loss in a Model of Postmenopausal Osteoporosis through Adrenomedullin Inhibition. Front. Physiol. 2016, 7, 280.
- Goldstein, J.L.; Zhao, T.J.; Li, R.L.; Sherbet, D.P.; Liang, G.; Brown, M.S. Surviving starvation: Essential role of the ghrelin-growth hormone axis. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 121–127.
- Serrano, J.; Alonso, D.; Fernández, A.P.; Encinas, J.M.; López, J.C.; Castro-Blanco, S.; Fernández-Vizarra, P.; Richart, A.; Santacana, M.; Uttenthal, L.O.; et al. Adrenomedullin in the central nervous system. Microsc. Res. Tech. 2002, 57, 76–90.
- Miyashita, K.; Itoh, H.; Arai, H.; Suganami, T.; Sawada, N.; Fukunaga, Y.; Sone, M.; Yamahara, K.; Yurugi-Kobayashi, T.; Park, K.; et al. The neuroprotective and vasculo-neuro-regenerative roles of adrenomedullin in ischemic brain and its therapeutic potential. Endocrinology 2006, 147, 1642–1653.
- Saita, M.; Shimokawa, A.; Kunitake, T.; Kato, K.; Hanamori, T.; Kitamura, K.; Eto, T.; Kannan, H. Central actions of adrenomedullin on cardiovascular parameters and sympathetic outflow in conscious rats. Am. J. Physiol. 1998, 274, 979–984.
- Kis, B.; Abrahám, C.S.; Deli, M.A.; Kobayashi, H.; Niwa, M.; Yamashita, H.; Busija, D.W.; Ueta, Y. Adrenomedullin, an autocrine mediator of blood-brain barrier function. Hypertens Res. 2003, 26, S61–S70.
- Fernandez, A.P.; Serrano, J.; Tessarollo, L.; Cuttitta, F.; Martinez, A. Lack of adrenomedullin in the mouse brain results in behavioral changes, anxiety, and lower survival under stress conditions. Proc. Natl. Acad. Sci. USA 2008, 105, 12581–12586.
- Fernandez, A.P.; Serrano, J.; Martinez-Murillo, R.; Martinez, A. Lack of Adrenomedullin in the Central Nervous System Results in Apparently Paradoxical Alterations on Pain Sensitivity. Endocrinology 2010, 151, 4908–4915.
- Gröschl, M.; Wendler, O.; Topf, H.G.; Bohlender, J.; Köhler, H. Significance of salivary adrenomedullin in the maintenance of oral health: Stimulation of oral cell proliferation and antibacterial properties. Regul. Pept. 2009, 154, 16–22.
- Hiroshima, Y.; Bando, M.; Kataoka, M.; Inagaki, Y.; Herzberg, M.C.; Ross, K.F.; Hosoi, K.; Nagata, T.; Kido, J.-I. Regulation of antimicrobial peptide expression in human gingival keratinocytes by interleukin-1α. Arch. Oral. Biol. 2011, 56, 761–767.
- McLachlan, J.L.; Smith, A.J.; Bujalska, I.J.; Cooper, P.R. Gene expression profiling of pulpal tissue reveals the molecular complexity of dental caries. Biochim. Biophys. Acta 2005, 1741, 271–281.
- Wang, H.; Tomikawa, M.; Jones, M.K.; Sarfeh, I.J.; Tarnawski, A.S. Ethanol injury triggers activation of adrenomedullin and its receptor genes in gastric mucosa. Dig. Dis. Sci. 1999, 44, 1390–1400.
- Fukuda, K.; Tsukada, H.; Oya, M.; Onomura, M.; Kodama, M.; Nakamura, H.; Hosokawa, M.; Seino, Y. Adrenomedullin promotes epithelial restitution of rat and human gastric mucosa in vitro. Peptides 1999, 20, 127–132.
- Sakata, J.; Asada, Y.; Shimokubo, T.; Kitani, M.; Inatsu, H.; Kitamura, K.; Kangawa, K.; Matsuo, H.; Sumiyoshi, A.; Eto, T. Adrenomedullin in the gastrointestinal tract. Distribution and gene expression in rat and augmented gastric adrenomedullin after fasting. J. Gastroenterol. 1998, 33, 828–834.
- Rossowski, W.J.; Jiang, N.Y.; Coy, D.H. Adrenomedullin, amylin, calcitonin gene-related peptide and their fragments are potent inhibitors of gastric acid secretion in rats. Eur. J. Pharmacol. 1997, 336, 51–63.
- Rossowski, W.J.; Cheng, B.L.; Jiang, N.Y.; Coy, D.H. Examination of somatostatin involvement in the inhibitory action of GIP, GLP-1, amylin and adrenomedullin on gastric acid release using a new SRIF antagonist analogue. Br. J. Pharmacol. 1998, 125, 1081–1087.
- Hirsch, A.B.; McCuen, R.W.; Arimura, A.; Schubert, M.L. Adrenomedullin stimulates somatostatin and thus inhibits histamine and acid secretion in the fundus of the stomach. Regul. Pept. 2003, 110, 189–195.
- Clementi, G.; Caruso, A.; Cutuli, V.M.; Mangano, N.G.; Salomone, S.; Lempereur, L.; Prato, A.; Matera, M.; Amico-Roxas, M. Gastroprotective effect of adrenomedullin administered subcutaneously in the rat. Peptides 2002, 23, 1149–1153.
- Salomone, S.; Caruso, A.; Cutuli, V.M.; Mangano, N.G.; Prato, A.; Amico-Roxas, M.; Bianchi, A.; Clementi, G. Effects of adrenomedullin on the contraction of gastric arteries during reserpine-induced gastric ulcer. Peptides 2003, 24, 117–122.
- Martínez, V.; Cuttitta, F.; Taché, Y. Central action of adrenomedullin to inhibit gastric emptying in rats. Endocrinology 1997, 138, 3749–3755.
- Holzer-Petsche, U.; Seitz, H.; Lembeck, F. Effect of capsaicin on gastric corpus smooth muscle of the rat in vitro. Eur. J. Pharmacol. 1989, 162, 29–36.
- Ebert, E.C. The thyroid and the gut. J. Clin. Gastroenterol. 2010, 44, 402–406.
- Mulder, H.; Ahren, B.; Karlsson, S.; Sundler, F. Adrenomedullin: Localization in the gastrointestinal tract and effects on insulin secretion. Regul. Pept. 1996, 62, 107–112.
- Hussain, S.; Miyazawa, R.; Tomomasa, T.; Kaneko, H.; Takahashi, A.; Watanabe, T.; Arakawa, H.; Morikawa, A. Possible involvement of adrenomedullin in lipopolysaccharide-induced small-intestinal motility changes in conscious rats. J. Gastroenterol. 2005, 40, 1123–1129.
- Zhou, M.; Chaudry, I.H.; Wang, P. The small intestine is an important source of adrenomedullin release during polymicrobial sepsis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 281, 654–660.
- Fukuda, K.; Tsukada, H.; Onomura, M.; Saito, T.; Kodama, M.; Nakamura, H.; Taniguchi, T.; Tominaga, M.; Hosokawa, M.; Seino, Y. Effect of adrenomedullin on ion transport and muscle contraction in rat distal colon. Peptides 1998, 19, 1043–1047.
- Kiyomizu, A.; Kitamura, K.; Kawamoto, M.; Eto, T. Distribution and molecular forms of adrenomedullin and proadrenomedullin N-terminal 20 peptide in the porcine gastrointestinal tract. J. Gastroenterol. 2001, 36, 18–23.
- Kravtsov, G.M.; Hwang, I.S.; Tang, F. The inhibitory effect of adrenomedullin in the rat ileum: Cross-talk with beta3-adrenoceptor in the serotonin-induced muscle contraction. J. Pharmacol. Exp. Ther. 2004, 308, 241–248.
- Takahashi, T. Interdigestive migrating motor complex -its mechanism and clinical importance. J. Smooth Muscle Res. 2013, 49, 99–111.
- Fernández de Arcaya, I.; Lostao, M.P.; Martínez, A.; Berjón, A.; Barber, A. Effect of adrenomedullin and proadrenomedullin N-terminal 20 peptide on sugar transport in the rat intestine. Regul. Pept. 2005, 129, 147–154.
- Martínez, A.; Cuttitta, F.; Teitelman, G. Expression pattern for adrenomedullin during pancreatic development in the rat reveals a common precursor with other endocrine cell types. Cell Tissue Res. 1998, 293, 95–100.
- Martínez, A.; Weaver, C.; López, J.; Bhathena, S.J.; Elsasser, T.H.; Miller, M.J.; Moody, T.W.; Unsworth, E.J.; Cuttitta, F. Regulation of insulin secretion and blood glucose metabolism by adrenomedullin. Endocrinology 1996, 137, 2626–2632.
- Sekine, N.; Takano, K.; Kimata-Hayashi, N.; Kadowaki, T.; Fujita, T. Adrenomedullin inhibits insulin exocytosis via pertussis toxin-sensitive G protein-coupled mechanism. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E9–E14.
- Martínez, A.; Pío, R.; López, J.; Cuttitta, F. Expression of the adrenomedullin binding protein, complement factor H, in the pancreas and its physiological impact on insulin secretion. J. Endocrinol. 2001, 170, 503–511.
- Tsuchida, T.; Ohnishi, H.; Tanaka, Y.; Mine, T.; Fujita, T. Inhibition of stimulated amylase secretion by adrenomedullin in rat pancreatic acini. Endocrinology 1999, 140, 865–870.
- Wan, X.; Song, M.; Wang, A.; Zhao, Y.; Wei, Z.; Lu, Y. Microbiome Crosstalk in Immunotherapy and Antiangiogenesis Therapy. Front. Immunol. 2021, 12, 747914.
- Trakman, G.L.; Fehily, S.; Basnayake, C.; Hamilton, A.L.; Russell, E.; Wilson-O’Brien, A.; Kamm, M.A. Diet and gut microbiome in gastrointestinal disease. J. Gastroenterol. Hepatol. 2021. online ahead of print.
- Martínez, A.; Elsasser, T.H.; Muro-Cacho, C.; Moody, T.W.; Miller, M.J.; Macri, C.J.; Cuttitta, F. Expression of adrenomedullin and its receptor in normal and malignant human skin: A potential pluripotent role in the integument. Endocrinology 1997, 138, 5597–5604.
- Allaker, R.P.; Kapas, S. Adrenomedullin and mucosal defence: Interaction between host and microorganism. Regul. Pept. 2003, 112, 147–152.
- Kishikawa, H.; Nishida, J.; Ichikawa, H.; Kaida, S.; Morishita, T.; Miura, S.; Hibi, T. Lipopolysaccharides stimulate adrenomedullin synthesis in intestinal epithelial cells: Release kinetics and secretion polarity. Peptides 2009, 30, 906–912.
- Matheson, P.J.; Mays, M.P.; Hurt, R.T.; Harris, P.D.; Garrison, R.N. Adrenornedullin is increased in the portal circulation during chronic sepsis in rats. Am. J. Surg. 2003, 186, 519–525.
- Walsh, T.J.; Martinez, A.; Peter, J.; Unsworth, E.; Cuttitta, F. Antimicrobial activity of adrenomedullin and its gene-related peptides. Clin. Infect. Dis. 1996, 23, 96.
- Allaker, R.P.; Zihni, C.; Kapas, S. An investigation into the antimicrobial effects of adrenomedullin on members of the skin, oral, respiratory tract and gut microflora. FEMS Immunol. Med. Microbiol. 1999, 23, 289–293.
- Kapas, S.; Bansal, A.; Bhargava, V.; Maher, R.; Malli, D.; Hagi-Pavli, E.; Allaker, R.P. Adrenomedullin expression in pathogen-challenged oral epithelial cells. Peptides 2001, 22, 1485–1489.
- Marutsuka, K.; Nawa, Y.; Asada, Y.; Hara, S.; Kitamura, K.; Eto, T.; Sumiyoshi, A. Adrenomedullin and proadrenomudullin N-terminal 20 peptide (PAMP) are present in human colonic epithelia and exert an antimicrobial effect. Exp. Physiol. 2001, 86, 543–545.
- Allaker, R.P.; Grosvenor, P.W.; McAnerney, D.C.; Sheehan, B.E.; Srikanta, B.H.; Pell, K.; Kapas, S. Mechanisms of adrenomedullin antimicrobial action. Peptides 2006, 27, 661–666.
This entry is offline, you can click here to edit this entry!
