口蹄疫(FMD)是一种急性和高度传染性疾病,会影响到诸如猪和牛之类的蹄类动物。导致FMD的病原体称为FMD病毒(FMDV),这是一种单链正义RNA病毒,在Picornaviridae家族中被归类为Aphthovirus属。
- foot-and-mouth disease virus
- immune escape
- innate immune response
- Interferon
- protein interaction
- viral protein
Note:All the information in this draft can be edited by authors. And the entry will be online only after authors edit and submit it.
1. Introduction
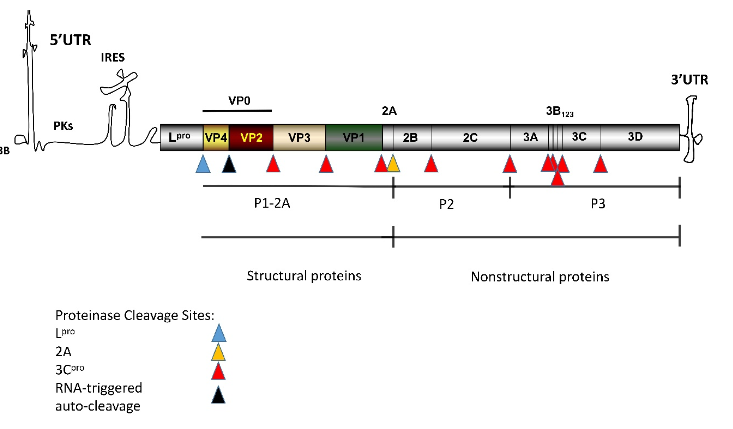
Foot-and-mouth disease (FMD) is an acute and highly contagious disease affecting thecloven-hoofed animals, such as pigs and cattle. The pathogen that causes FMD is known as FMD virus(FMDV), a single-stranded positive-sense RNA virus that is classified into the genus Aphthovirus inthe family Picornaviridae[1][2]. The pathogen causes vesicular disease of mouth and feet in susceptibleanimals[3]. The high mutation rate of the genome of FMDV and the rapid proliferation has led to therapid evolution of the virus and the formation of seven main serotypes [4][5][6]. The antigenic diversityamong the serotypes poses challenges to the research of efficient and cross-protective vaccines [8].The genome of FMDV contains an open reading frame (ORF) that encodes a polyprotein precursor,and it is cleaved into four structural proteins and 10 non-structural proteins by viral autoproteases andhost protease [7](Figure 1).Upon infection of the host, a virus will face the attack from the host’s immune response. In thelong-term battle with the host immune response, the virus has evolved and developed a series ofimmune escape mechanisms to overcome the killing and inhibition from the host immune system.The mechanism of virus immune escape can be divided into three categories: (1) enable the virus toavoid the recognition of humoral immune response; (2) interfere with the function of cellular immuneresponse; (3) interfere with the host’s immune response to the virus [8]. All these strategies would beexploited by the virus for replication and spreading to other hosts.As a highly contagious and fast-spreading virus, FMDV has multiple ways to evade the killing bythe immune system[9], which makes it difficult for controlling the virus. Viral capsid protein VP1 and leading protein Lpro can inhibit the production of interferon (IFN) and innate immune responseby interacting with soluble resistance-related calcium-binding protein (sorcin) or host transcriptionfactor ADNP [10][11]. Recently, new mechanisms and functions of FMDV proteins inhibiting innateimmunity have been discovered. DDX56 (a kind of RNA helicase), participate in RNA metabolism andribosome synthesis is reported to involve in this new mechanism. The interaction between FMDV3A and DDX56 suppresses the host innate immunity by reducing the phosphorylation of IRF3 [12].In addition, nucleotide-binding oligomerization domain 2 (NOD2), a member of the nucleotide-bindingoligomerization domain-like receptor (NLR) family [13], activates the NF-κB and IFN-βsignalingpathways during FMDV infection and inhibits the replication of FMDV in infected cells [14]. FMDV 2B,2C, and 3Cproinhibit the expression of NOD2 protein, which antagonizes the antiviral response [14].Reportedly, multiple structural and non-structural proteins of FMDV escape the killing of the hostimmune system. This review summrized the molecular mechanisms of immune evasion caused byFMDV proteins. The present study aimed to fill the gaps of knowledge on FMDV immune evasionmechanism, providing the basis for the prevention and control strategies for FMDV.
Figure 1.Schematic of the genome and polypeptide processing of FMDV [2][7]. The FMDV genomecontains an ORF of about 7 kbp, indicated by the shaded rectangle. Each region within the ORFrectangle represents a single protein. The flank of ORF is a long 5′untranslated region (5′-UTR) and ashort 3′-UTR. 3B covalently binds to the 5′-end.
2. Prospects and Future Directions
FMDV是一种具有高度传染性的病毒,几乎感染了所有偶蹄类动物,在脚和嘴上显示出囊泡,在粘膜上出现皮肤侵蚀,发烧,体重减轻,起搏和流涎,严重威胁着畜牧业的发展。但是,FMDV除了引起急性感染和疾病外,在某些情况下还可能是无症状的携带者,这可能会导致口蹄疫的再次爆发,使预防和控制工作面临挑战且成本高昂。高传染性,广泛的地理分布,广泛的寄主范围,没有血清型交叉保护的短期免疫,多种传播方式和持续感染使控制和根除这种疾病相当困难。因此,研究控制FMDV逃避免疫力的分子机制对于控制流行病势在必行。免疫系统包括先天免疫力和获得性免疫力,这是抵抗病原微生物入侵,监视和清除异物的主要保护系统。在感染初期抑制免疫系统的功能,从而使病毒可以在呼吸系统中迅速增殖并传播到其自然感染部位[ 15 ]。就逃避体液免疫系统而言,FMDV的每种血清型都容易发生抗原变异,使病毒从中和抗体中逃脱[ 16 ]。在抑制细胞免疫反应方面,FMDV感染可引起宿主淋巴细胞减少,并伴有严重病毒血症,最终将导致T细胞的破坏,FMDV感染会抑制树突状细胞的功能,削弱树突状细胞的加工能力。抗原[143,144]。先前的研究表明,FMDV感染后30分钟,细胞表面的MHC I类分子表达被抑制,这表明被FMDV感染的细胞将立即失去向T淋巴细胞呈递MHC-I相关病毒肽的能力。将有助于病毒从宿主的细胞毒性免疫反应中逃脱。限制NK细胞介导的杀伤作用也是FMDV逃避细胞免疫应答的重要机制。[ 17 ]。令人惊讶的是,从感染猪中分离出的NK细胞不能分泌IFN-γ [ 18 ]。FMDV干扰免疫效果和抑制先天免疫的研究已广泛开展。FMDV(Lpro,2B,3A,3B,3C)的某些蛋白质可以直接或间接作用于视黄酸诱导的基因I样受体(RLR),以抑制先天性免疫[ 19 ] [ 20 ] [ 21 ] [ 22 ] [ 23 ]。FMDV VP0,VP3、3A和3B在转录或蛋白质水平上降低连接蛋白VISA的表达[ 23 ] [24 ] [ 25 ]。FMDV Lpro,VP0,VP1、2B和3A可以直接或间接靶向IRF3以抑制干扰素产生 [ 21 ] [ 23 ] [ 26 ]。VP3和3C蛋白抑制JAK-STAT信号传导途径,从而抑制ISG的产生 [ 27 ] [ 28 ]。FMDV蛋白Lproand 3C通过切割宿主转录和翻译的相关因子来抑制抗病毒分子的合成 [ 11 ] [ 29 ] [ 30 ] [ 31 ] [ 32]。此外,有趣的是,Lpro蛋白不仅可以诱导细胞凋亡,而且可以抑制宿主细胞凋亡并促进病毒复制,这是通过阻断α-IFN的翻译并抑制PKR合成来实现的[ 33 ]。FMDV蛋白VP2和2C可以通过调节自噬来促进病毒复制[ 34 ] [ 35 ]。这些机制为FMDV的快速转录和翻译提供了机会。在以前的FMDV研究中,HEK293细胞因其高转染效率而被广泛用于体外实验。但是,HEK293细胞不是FMDV易感细胞,并且HEK293细胞和FMDV易感细胞之间存在物种差异。因此,将HEK293细胞用于FMDV相关研究有一定的局限性。
3.结论
总之,FMDV已经进化出多种方法来逃避与宿主免疫系统的长期战斗中的免疫反应。尽管FMDV的免疫逃逸研究取得了许多突破,但仍阐明了受FMDV影响的宿主免疫的许多机制,还需要进一步探讨FMDV蛋白与宿主蛋白之间的相互作用。除了病毒与宿主蛋白之间的相互作用外,探索多种病毒蛋白协同抑制免疫反应的机制对于开发特定药物和新疫苗也具有重要意义。先前的研究主要集中于FMDV对先天免疫的影响。但是,关于获得性免疫的研究很少,有待进一步补充。也,
This entry is adapted from the peer-reviewed paper 10.3390/pathogens9090729
References
- Soren Alexandersen; N. Mowat; Foot-and-Mouth Disease: Host Range and Pathogenesis. Current Topics in Microbiology and Immunology / Ergebnisse der Microbiologie und Immunitätsforschung 2005, 288, 9-42, 10.1007/3-540-27109-0_2.
- Syed M. Jamal; Graham J. Belsham; Foot-and-mouth disease: past, present and future. Veterinary Research 2013, 44, 116-116, 10.1186/1297-9716-44-116.
- Nick J. Knowles; A R Samuel; Molecular epidemiology of foot-and-mouth disease virus. Virus Research 2003, 91, 65-80, 10.1016/s0168-1702(02)00260-5.
- Daniel T. Haydon; A.R Samuel; N.J Knowles; The generation and persistence of genetic variation in foot-and-mouth disease virus. Preventive Veterinary Medicine 2001, 51, 111-124, 10.1016/s0167-5877(01)00210-0.
- E. Domingo; Nonia Pariente; A. Airaksinen; C. González-Lopez; S. Sierra; M. Herrera; Ana Grande-Pérez; P. R. Lowenstein; S. C. Manrubia; E. Lázaro; et al. Foot-and-Mouth Disease Virus Evolution: Exploring Pathways Towards Virus Extinction. Inducible Lymphoid Organs 2005, 288, 149-173, 10.1007/3-540-27109-0_7.
- E. Domingo; Carmen M Ruiz-Jarabo; Saleta Sierra; Armando Arias; Nonia Pariente; Eric Baranowski; Cristina Escarmis; Emergence and selection of RNA virus variants: memory and extinction.. Virus Research 2002, 82, 39-44, 10.1016/s0168-1702(01)00385-9.
- Peter W. Mason; Marvin J Grubman; Barry Baxt; Molecular basis of pathogenesis of FMDV. Virus Research 2003, 91, 9-32, 10.1016/s0168-1702(02)00257-5.
- Mireille T. Vossen; Ellen M. Westerhout; Cecilia Söderberg-Naucler; Emmanuel J. H. J. Wiertz; Viral immune evasion: a masterpiece of evolution. Immunogenetics 2002, 54, 527-542, 10.1007/s00251-002-0493-1.
- William T. Golde; Charles K. Nfon; Felix N. Toka; Immune evasion during foot‐and‐mouth disease virus infection of swine. Immunological Reviews 2008, 225, 85-95, 10.1111/j.1600-065X.2008.00672.x.
- Xiaying Li; Jianchang Wang; Jue Liu; Zhonghua Li; Yongqiang Wang; Yanfei Xue; Xiaoqi Li; Hong Cao; Shijun J. Zheng; Engagement of soluble resistance-related calcium binding protein (sorcin) with foot-and-mouth disease virus (FMDV) VP1 inhibits type I interferon response in cells. Veterinary Microbiology 2013, 166, 35-46, 10.1016/j.vetmic.2013.04.028.
- Gisselle N. Medina; Giselle M. Knudsen; Alexander L. Greninger; Anna Kloc; Fayna Díaz-San Segundo; Elizabeth Rieder; Marvin J. Grubman; Joseph L. DeRisi; Teresa De Los Santos; Interaction between FMDV Lpro and transcription factor ADNP is required for optimal viral replication. Virology 2017, 505, 12-22, 10.1016/j.virol.2017.02.010.
- Shao-Zu Fu; Wen-Ping Yang; Yi Ru; Ke-Shan Zhang; Yong Wang; Xiang-Tao Liu; Dan Li; Haixue Zheng; DDX56 cooperates with FMDV 3A to enhance FMDV replication by inhibiting the phosphorylation of IRF3.. Cellular Signalling 2019, 64, 109393, 10.1016/j.cellsig.2019.109393.
- Ahmed Sabbah; Te Hung Chang; Rosalinda Harnack; Victoria Frohlich; Kaoru Tominaga; Peter H. Dube; Yan Xiang; Santanu Bose; Activation of innate immune antiviral responses by Nod2. Nature Immunology 2009, 10, 1073-1080, 10.1038/ni.1782.
- Huisheng Liu; Zixiang Zhu; Qiao Xue; Fan Yang; Weijun Cao; Keshan Zhang; Xiangtao Liu; Haixue Zheng; Foot-and-Mouth Disease Virus Antagonizes NOD2-Mediated Antiviral Effects by Inhibiting NOD2 Protein Expression. Journal of Virology 2019, 93, 93, 10.1128/jvi.00124-19.
- Carolina Stenfeldt; Fayna Diaz-San Segundo; Teresa De Los Santos; Luis L. Rodriguez; Jonathan Arzt; The Pathogenesis of Foot-and-Mouth Disease in Pigs. Frontiers in Veterinary Science 2016, 3, 12, 10.3389/fvets.2016.00041.
- Carolina Stenfeldt; Juan M. Pacheco; M.V. Borca; Luis L. Rodriguez; Jonathan Arzt; Morphologic and phenotypic characteristics of myocarditis in two pigs infected by foot-and mouth disease virus strains of serotypes O or A. Acta Veterinaria Scandinavica 2014, 56, 42-42, 10.1186/s13028-014-0042-6.
- Felix N. Toka; Charles Nfon; Harry Dawson; William T. Golde; Natural Killer Cell Dysfunction during Acute Infection with Foot-and-Mouth Disease Virus. Clinical and Vaccine Immunology 2009, 16, 1738-1749, 10.1128/cvi.00280-09.
- Felix N. Toka; William T. Golde; Cell mediated innate responses of cattle and swine are diverse during foot-and-mouth disease virus (FMDV) infection: A unique landscape of innate immunity. Immunology Letters 2013, 152, 135-143, 10.1016/j.imlet.2013.05.007.
- Miguel Rodríguez-Pulido; María T. Sánchez-Aparicio; Encarnación Martínez-Salas; Adolfo García-Sastre; Francisco Sobrino; Margarita Sáiz; Innate immune sensor LGP2 is cleaved by the Leader protease of foot-and-mouth disease virus. PLOS Pathogens 2018, 14, e1007135, 10.1371/journal.ppat.1007135.
- Zixiang Zhu; Guoqing Wang; Fan Yang; Weijun Cao; Ruoqing Mao; Xiaoli Du; Xiangle Zhang; Chuntian Li; Dan Li; Keshan Zhang; et al. Foot-and-Mouth Disease Virus Viroporin 2B Antagonizes RIG-I-Mediated Antiviral Effects by Inhibition of Its Protein Expression. Journal of Virology 2016, 90, 11106-11121, 10.1128/jvi.01310-16.
- Zixiang Zhu; Chuntian Li; Xiaoli Du; Guoqing Wang; Weijun Cao; Fan Yang; Huanhuan Feng; Xiangle Zhang; Zhengwang Shi; Huanan Liu; et al. Foot-and-mouth disease virus infection inhibits LGP2 protein expression to exaggerate inflammatory response and promote viral replication.. Cell Death & Disease 2017, 8, e2747-e2747, 10.1038/cddis.2017.170.
- Ming Li; Ting Xin; Xintao Gao; Jing Wu; Xixi Wang; Lichun Fang; Xiukun Sui; Hongfei Zhu; Shangjin Cui; Xiaoyu Guo; et al. Foot-and-mouth disease virus non-structural protein 2B negatively regulates the RLR-mediated IFN-β induction. Biochemical and Biophysical Research Communications 2018, 504, 238-244, 10.1016/j.bbrc.2018.08.161.
- Dan Li; Caoqi Lei; Zhisheng Xu; Fan Yang; Huanan Liu; Zixiang Zhu; Shu Li; Xiangtao Liu; Hong-Bing Shu; Haixue Zheng; et al. Foot-and-mouth disease virus non-structural protein 3A inhibits the interferon-β signaling pathway. Scientific Reports 2016, 6, 21888, 10.1038/srep21888.
- Dan Li; Jing Zhang; Wenping Yang; Yanchun He; Yi Ru; Shaozu Fu; Lulu Li; Xiangtao Liu; Haixue Zheng; Poly (rC) binding protein 2 interacts with VP0 and increases the replication of the foot-and-mouth disease virus.. Cell Death & Disease 2019, 10, 516, 10.1038/s41419-019-1751-6.
- Dan Li; Wenping Yang; Fan Yang; Huanan Liu; Zixiang Zhu; Kaiqi Lian; Caoqi Lei; Shu Li; Xiangtao Liu; Haixue Zheng; et al. The VP3 structural protein of foot‐and‐mouth disease virus inhibits the IFN‐β signaling pathway. The FASEB Journal 2016, 30, 1757-1766, 10.1096/fj.15-281410.
- Dang Wang; Liurong Fang; Rui Luo; Rui Ye; Ying Fang; Lilan Xie; Huanchun Chen; Shaobo Xiao; Foot-and-mouth disease virus leader proteinase inhibits dsRNA-induced type I interferon transcription by decreasing interferon regulatory factor 3/7 in protein levels. Biochemical and Biophysical Research Communications 2010, 399, 72-78, 10.1016/j.bbrc.2010.07.044.
- Dan Li; Jin Wei; Fan Yang; Hua-Nan Liu; Zi-Xiang Zhu; Wei-Jun Cao; Shu Li; Xiang-Tao Liu; Haixue Zheng; Hong-Bing Shu; et al. Foot-and-mouth disease virus structural protein VP3 degrades Janus kinase 1 to inhibit IFN-γ signal transduction pathways. Function of a membrane-embedded domain evolutionarily multiplied in the GPI lipid anchor pathway proteins PIG-B, PIG-M, PIG-U, PIG-W, PIG-V, and PIG-Z 2016, 15, 850-860, 10.1080/15384101.2016.1151584.
- Yijun Du; Jingshan Bi; Jiyu Liu; Xing Liu; Xiangju Wu; Ping Jiang; Dongwan Yoo; Yongguang Zhang; Jiaqiang Wu; Renzhong Wan; et al. 3Cpro of Foot-and-Mouth Disease Virus Antagonizes the Interferon Signaling Pathway by Blocking STAT1/STAT2 Nuclear Translocation. Journal of Virology 2014, 88, 4908-4920, 10.1128/jvi.03668-13.
- Anne-Claude Gingras; Brian Raught; Nahum Sonenberg; eIF4 Initiation Factors: Effectors of mRNA Recruitment to Ribosomes and Regulators of Translation. Annual Review of Biochemistry 1999, 68, 913-963, 10.1146/annurev.biochem.68.1.913.
- Walter Glaser; Regina Cencic; Tim Skern; Robert P. Fadden; Amparo Ruiz; Timothy Haystead; Joaquı́n Ariño; Eulàlia De Nadal; Foot-and-Mouth Disease Virus Leader Proteinase. Journal of Biological Chemistry 2001, 276, 35473-35481, 10.1074/jbc.m104192200.
- Graham J. Belsham; Gerald M. McInerney; Natalie Ross-Smith; Foot-and-Mouth Disease Virus 3C Protease Induces Cleavage of Translation Initiation Factors eIF4A and eIF4G within Infected Cells. Journal of Virology 2000, 74, 272-280, 10.1128/JVI.74.1.272-280.2000.
- Paul Lawrence; Elizabeth A. Schafer; Elizabeth Rieder; The nuclear protein Sam68 is cleaved by the FMDV 3C protease redistributing Sam68 to the cytoplasm during FMDV infection of host cells. Virology 2012, 425, 40-52, 10.1016/j.virol.2011.12.019.
- Chelsea M. Hull; Philip C. Bevilacqua; Discriminating Self and Non-Self by RNA: Roles for RNA Structure, Misfolding, and Modification in Regulating the Innate Immune Sensor PKR. Accounts of Chemical Research 2016, 49, 1242-1249, 10.1021/acs.accounts.6b00151.
- Peng Sun; Shumin Zhang; Xiaodong Qin; Xingni Chang; Xiaorui Cui; Haitao Li; Shuaijun Zhang; Huanhuan Gao; Penghua Wang; Zhidong Zhang; et al. Foot-and-mouth disease virus capsid protein VP2 activates the cellular EIF2S1-ATF4 pathway and induces autophagy via HSPB1.. Autophagy 2018, 14, 336-346, 10.1080/15548627.2017.1405187.
- Douglas P. Gladue; V. O'donnell; R. Baker-Branstetter; L. G. Holinka; Juan M. Pacheco; I. Fernandez-Sainz; Z. Lu; E. Brocchi; B. Baxt; M. E. Piccone; et al. Foot-and-Mouth Disease Virus Nonstructural Protein 2C Interacts with Beclin1, Modulating Virus Replication. Journal of Virology 2012, 86, 12080-12090, 10.1128/jvi.01610-12.