2. Insightful Analysis
The reduction of GO deposited on the fabric made the fabric electrically conductive. When the temperature of the hot stage approached 220 °C, R diminished significantly but at 220 °C it leveled off within 1 min at the value of 0.1 MΩ/sq. During cooling and further storage at RT, R increased but finally stabilized after 24 h. Such behavior was observed by us previously [
12,
13,
32] due to partial reversibility of the reduction. Nevertheless, R of 5.4 MΩ/sq was achieved, showing the presence of conducting 3D-network of rGO on fiber surfaces.
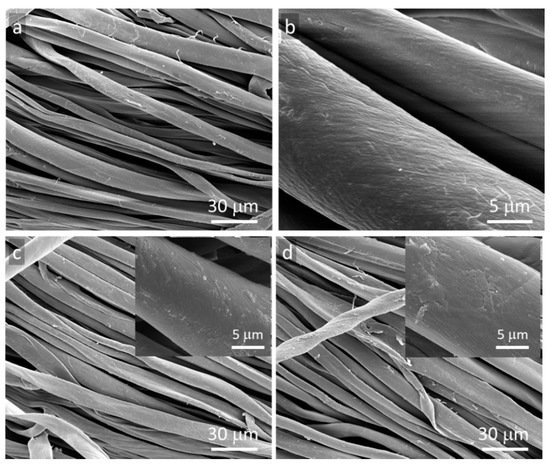
SEM micrographs of GO and rGO-coated fibers are shown in
Figure 1. Small thickness and very good adhesion to fiber surfaces make the particles hardly visible on the fibers [
13]. However, the areas without typical morphology of cotton fibers are discernible, shown in the insets, which are suggestive of GO or rGO thin layers.
Figure 1. SEM micrographs of cotton fabric: (a,b) neat, (c) GO-coated, (d) rGO-coated.
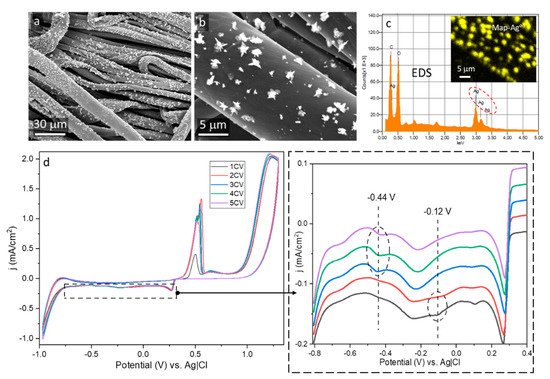
Reduction of Ag
+ ions occurred on the rGO-coated surfaces, combined with nucleation and growth of Ag° particles, which are visible in SEM micrographs in
Figure 2a,b. EDS analysis confirmed the presence of Ag° particles on rGO-coated surface, as shown in
Figure 2c. Reaction of silver nitrate with sodium citrate resulted in the formation of silver citrate complex stabilized by carboxyl groups of citric acid, which prevented aggregation of Ag° particles and enabled development of particles immobilized on the surface [
33]. The most effective complexing occurs when [citrate]/[Ag
+] >> 1 [
34]. Others [
35] studied GO electrochemistry and found that the reduction reactions occurred on edges of GO plates. Thus, we postulate that the reduction of the complex and the formation of Ag° particles, through nucleation and growth, could occur on edges or defects of rGO plates.
Figure 2. Ag°/rGO-coated cotton fabric: (a,b) SEM micrographs, (c) EDS Ag mapping and spectrum, (d) cyclic voltammetry profiles.
The voltammetry profile during the first and the second cycle, shown in
Figure 2d, exhibits one redox weak peak at −0.12 V attributed by others to reduction of hydroxyl groups [
36]. Most probably, it was related to residual hydroxyl groups of rGO, remaining due to its incomplete thermal reduction. Further reactions postulated in [
36] were not observed due to a small number of other functional groups on rGO surface and competitive reactions involving Ag
+ ions. During the next three cycles, a peak at −0.44 V evidenced the reduction of Ag
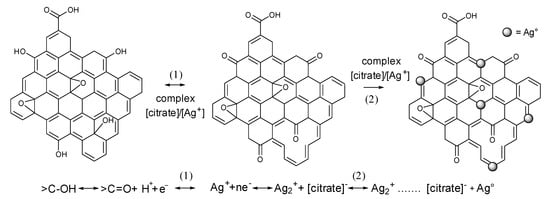
+ ions. The proposed mechanism of the formation of the Ag° particles is shown in
Scheme 1.
Scheme 1. Proposed mechanism of Ag° formation on rGO deposited on cotton fiber surfaces.
Based on the results of TGA experiments, by taking into account the residue weight at 900 °C of Ag°/rGO-modified fabric and rGO-modified fabric, the Ag content of 0.4 wt.% was determined. The successful deposition of Ag° particles confirmed the formation of the electroconductive rGO network on fiber surfaces and indicated that rGO-coated fabrics can serve as electrodes in cyclic voltammetry. R of the fabric did not change after the electrochemical modification and was equal to 5.35 MΩ/sq because Ag° was deposited on the rGO as separate particles, which did not form any continuous network. However, the Ag°/rGO-modified fabric became superhydrophobic with WCA of 161°, due to the presence of hydrophobic Ag° particles on the surface, causing the lotus effect.
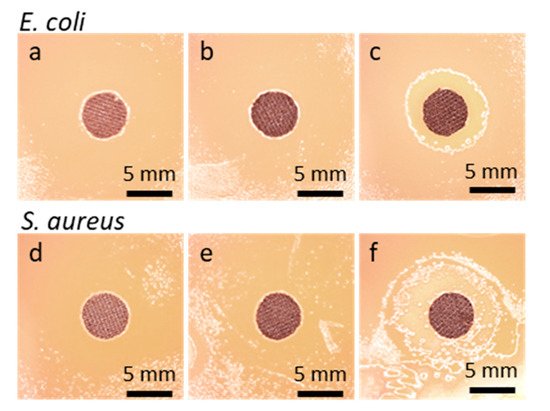
As seen in Figure 3, no bacterial growth inhibition zone was found around GO-coated or rGO-coated fabric specimens. The growth inhibition zones of about 9 mm and 12 mm for Gram-positive Staphylococcus aureus and Gram-negative Escherichia coli, respectively, were only observed around the specimens of Ag°/rGO-coated fabric.
Figure 3. Disk specimens of cotton fabric on bacteria inoculated agar after 48 h incubation: E. coli (a) GO-coated, (b) rGO-coated, (c) Ag°/rGO-coated; S. aureus, (d) GO-coated, (e) rGO-coated, (f) Ag°/rGO-coated.
Ag° particles exhibit non-specific bacterial toxicity that impedes the bacteria resistance development and also broadens the spectrum of antibacterial activity [
37]. The main activity mechanisms of metal nanoparticles or ions include attraction to bacterial cell walls, disruption of the cell walls increasing their permeability, and disruption of cell functions [
23,
24,
25,
37,
38].
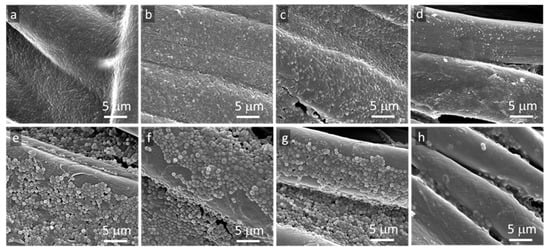
The absence of bacterial growth inhibition zones around specimens does not exclude their antibacterial activity in direct contact with microorganisms. Therefore, SEM was used to analyze the bottom surfaces of the tested fabric specimens. SEM micrographs of bottom surfaces of cotton fabric specimens in Figure 4, modified and unmodified, show that not only the neat fibers but also GO and rGO-coated fibers were inhabited by both tested bacterial strains.
Figure 4. SEM micrographs of bottom surfaces of cotton fabric specimens removed from agar after 48 h incubation: E. coli (a) neat fabric, (b) GO-coated, (c) rGO-coated, (d) Ag°/rGO-coated, S.aureus (e) neat fabric, (f) GO-coated, (g) rGO-coated, (h) Ag°/rGO-coated.
According to the literature, there are three basic mechanisms of antibacterial activity of GO. The first one, the most commonly observed, is the effect of sharp edges, known as nanoblade effect. The nanoblades cut cell membranes causing their damage leading to the death of bacteria [
16,
17,
18]. The second one is oxidative stress caused by reactive oxygen species, leading to bacteria DNA damage and mitochondrial dysfunction [
39]. The third mechanism reported, observed rather in solutions than in coatings, is wrapping or trapping of bacteria by GO flakes, which causes their isolation from the environment [
40]. The main mechanisms of antibacterial activity of rGO are related to the nanoblade effect and oxidative stress [
15]. In the current study, GO or rGO flakes adhering to cotton fibers and lying flat on them did not expose sharp edges, and no wrapping or trapping of bacteria was observed by SEM. Therefore, their antibacterial activity was greatly limited. To achieve electroconductivity, a sufficient amount of GO particles had to be deposited on the fiber surfaces to form a 3D-newtork, which was then made electroconductive by GO reduction. However, the formation of 3D-network did not require full coverage of fibers with GO or rGO platelets. The uncovered areas, without coating, were prone to bacteria colonization. In turn, the activity of Ag° particles is well known and includes the release of Ag
+ through oxidative dissolution, in which atmospheric O
2 dissolved in water plays a role of oxidizer [
38]. Due to the diffusion of thus formed ions, the antibacterial activity of Ag° modified surface does not require a close contact with microorganisms, which is reflected in the bacterial growth inhibition zones around the specimens in
Figure 3.