Skeletal muscle mass plays a critical role in a healthy lifespan by helping to regulate glucose homeostasis. As seen in sarcopenia, decreased skeletal muscle mass impairs glucose homeostasis, but it may also be caused by glucose dysregulation. Gut microbiota modulates lipopolysaccharide (LPS) production, short-chain fatty acids (SCFA), and various metabolites that affect the host metabolism, including skeletal muscle tissues, and may have a role in the sarcopenia etiology. The evidence presented in this entry suggests that loss of muscle mass and function are not an inevitable consequence of the aging process, and that dietary and lifestyle interventions may prevent or delay sarcopenia.
- skeletal muscle mass
- L-leucine
- glucose metabolism
- gut microbiome
- short-chain fatty acids
1. Introduction
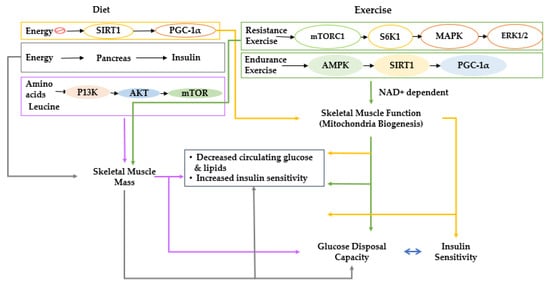
2. Overall View of Muscle Metabolism and the Factors That Control Muscle Growth
3. Age-Related Interactive Effects of Impaired Glucose Metabolism and Sarcopenia
3.1. Skeletal Muscle Mass Loss during Aging
3.2. Sarcopenia and Glucose Metabolism
4. Glucose Metabolism and Gut Microbiota
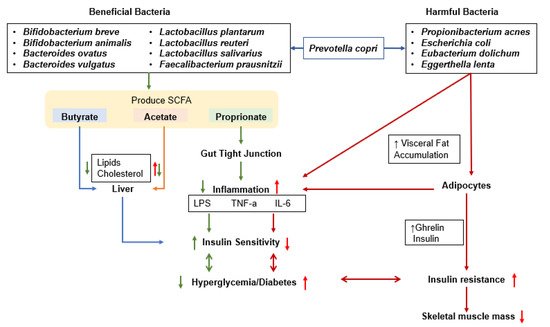
5. Effects of the Microbiome on Muscle Mass and the Development of Sarcopenia
5.1. Physical Activity, Sarcopenia, and Gut–Muscle Axis
5.2. Gut Microbiota, Sarcopenia, and Gut–Muscle Axis
5.3. Myokines, Sarcopenia, and Gut–Liver–Muscle Axis
6. BCAA Effects on Metabolism and Sarcopenia Host through the Gut–Muscle Axis
7. The Modulation of Dietary Intake and Lifestyles and Gut Microbiome to Promote Skeletal Muscle Mass and Prevent Sarcopenia
7.1. Calorie and Fat Intake, Gut Microbiota, and Skeletal Muscle Mass
7.2. Probiotic and Prebiotic Intakes, Gut Microbiota, and Skeletal Muscle Mass
8. Summary and Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/cells11030338
References
- Peterson, S.J.; Mozer, M. Differentiating Sarcopenia and Cachexia Among Patients With Cancer. Nutr. Clin. Pract. 2017, 32, 30–39.
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31.
- Lampignano, L.; Bortone, I.; Castellana, F.; Donghia, R.; Guerra, V.; Zupo, R.; De Pergola, G.; Di Masi, M.; Giannelli, G.; Lozupone, M.; et al. Impact of Different Operational Definitions of Sarcopenia on Prevalence in a Population-Based Sample: The Salus in Apulia Study. Int. J. Environ. Res. Public Health 2021, 18, 12979.
- Molino, S.; Dossena, M.; Buonocore, D.; Verri, M. Sarcopenic Obesity: An Appraisal of the Current Status of Knowledge and Management in Elderly People. J. Nutr. Health Aging 2016, 20, 780–788.
- Yaribeygi, H.; Farrokhi, F.R.; Butler, A.E.; Sahebkar, A. Insulin resistance: Review of the underlying molecular mechanisms. J. Cell. Physiol. 2019, 234, 8152–8161.
- Mesinovic, J.; Zengin, A.; De Courten, B.; Ebeling, P.R.; Scott, D. Sarcopenia and type 2 diabetes mellitus: A bidirectional relationship. Diab. Metab. Syndr. Obes. 2019, 12, 1057–1072.
- Mitchell, W.K.; Williams, J.; Atherton, P.; Larvin, M.; Lund, J.; Narici, M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front. Physiol. 2012, 3, 260.
- Lee, J.H.; Jun, H.-S. Role of Myokines in Regulating Skeletal Muscle Mass and Function. Front. Physiol. 2019, 10, 42.
- Klein, G.L. The effect of glucocorticoids on bone and muscle. Osteoporos. Sarcopenia 2015, 1, 39–45.
- Ji, L.L.; Yeo, D. Mitochondrial dysregulation and muscle disuse atrophy. F1000Research 2019, 8, F1000.
- Schakman, O.; Dehoux, M.; Bouchuari, S.; Delaere, S.; Lause, P.; Decroly, N.; Shoelson, S.E.; Thissen, J.P. Role of IGF-I and the TNFα/NF-κB pathway in the induction of muscle atrogenes by acute inflammation. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E729–E739.
- Novotny, S.A.; Warren, G.L.; Hamrick, M.W. Aging and the muscle-bone relationship. Physiology 2015, 30, 8–16.
- Nair, K.S. Aging muscle. Am. J. Clin. Nutr. 2005, 81, 953–963.
- Al Saedi, A.; Phu, S.; Vogrin, S.; Gunawardene, P.; Duque, G. Association between Circulating Osteoprogenitor Cells and Sarcopenia. Gerontology 2021, 1–6.
- Kim, G.; Kim, J.H. Impact of Skeletal Muscle Mass on Metabolic Health. Endocrinol. Metab. 2020, 35, 1–6.
- Park, S. Association between polygenetic risk scores related to sarcopenia risk and their interactions with regular exercise in a large cohort of Korean adults. Clin. Nutr. 2021, 40, 5355–5364.
- Tournadre, A.; Vial, G.; Capel, F.; Soubrier, M.; Boirie, Y. Sarcopenia. Jt. Bone Spine 2019, 86, 309–314.
- Romanello, V.; Sandri, M. Mitochondrial qaulity control and muscle mass maintenance. Front. Physiol. 2016, 6, 422.
- Lee, M.J.; Kim, E.-H.; Bae, S.-J.; Choe, J.; Jung, C.H.; Lee, W.J.; Kim, H.-K. Protective role of skeletal muscle mass against progression from metabolically healthy to unhealthy phenotype. Clin. Endocrinol. 2019, 90, 102–113.
- Kim, H.K.; Lee, M.J.; Kim, E.H.; Bae, S.J.; Kim, K.W.; Kim, C.H. Comparison of muscle mass and quality between metabolically healthy and unhealthy phenotypes. Obesity 2021, 29, 1375–1386.
- Volpi, E.; Nazemi, R.; Fujita, S. Muscle tissue changes with aging. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 405–410.
- Bosy-Westphal, A.; Müller, M.J. Identification of skeletal muscle mass depletion across age and BMI groups in health and disease--there is need for a unified definition. Int. J. Obes. 2015, 39, 379–386.
- Anker, S.D.; Morley, J.E.; von Haehling, S. Welcome to the ICD-10 code for sarcopenia. J. Cachexia Sarcopenia Muscle 2016, 7, 512–514.
- Buscemi, C.; Ferro, Y.; Pujia, R.; Mazza, E.; Boragina, G.; Sciacqua, A.; Piro, S.; Pujia, A.; Sesti, G.; Buscemi, S.; et al. Sarcopenia and Appendicular Muscle Mass as Predictors of Impaired Fasting Glucose/Type 2 Diabetes in Elderly Women. Nutrients 2021, 13, 1909.
- Haran, P.H.; Rivas, D.A.; Fielding, R.A. Role and potential mechanisms of anabolic resistance in sarcopenia. J. Cachexia Sarcopenia Muscle 2012, 3, 157–162.
- Lee, E.J.; Neppl, R.L. Influence of Age on Skeletal Muscle Hypertrophy and Atrophy Signaling: Established Paradigms and Unexpected Links. Genes 2021, 12, 688.
- Moro, T.; Brightwell, C.R.; Deer, R.R.; Graber, T.G.; Galvan, E.; Fry, C.S.; Volpi, E.; Rasmussen, B.B. Muscle Protein Anabolic Resistance to Essential Amino Acids Does Not Occur in Healthy Older Adults Before or After Resistance Exercise Training. J. Nutr. 2018, 148, 900–909.
- Paulussen, K.J.M.; McKenna, C.F.; Beals, J.W.; Wilund, K.R.; Salvador, A.F.; Burd, N.A. Anabolic Resistance of Muscle Protein Turnover Comes in Various Shapes and Sizes. Front. Nutr. 2021, 8, 615849.
- Dendup, T.; Feng, X.; Clingan, S.; Astell-Burt, T. Environmental Risk Factors for Developing Type 2 Diabetes Mellitus: A Systematic Review. Int. J. Environ. Res. Public Health 2018, 15, 78.
- Khodaeian, M.; Enayati, S.; Tabatabaei-Malazy, O.; Amoli, M.M. Association between Genetic Variants and Diabetes Mellitus in Iranian Populations: A Systematic Review of Observational Studies. J. Diabetes Res. 2015, 2015, 585917.
- Yu, L.; Zhou, X.; Duan, H.; Chen, Y.; Cui, S.; Guo, R.; Xue, Y.; Tian, F.; Zhao, J.; Zhang, H.; et al. Synergistic Protective Effects of Different Dietary Supplements Against Type 2 Diabetes via Regulating Gut Microbiota. J. Med. Food 2021, 24, 319–330.
- Chambers, E.S.; Preston, T.; Frost, G.; Morrison, D.J. Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Curr. Nutr. Rep. 2018, 7, 198–206.
- Anhê, F.F.; Barra, N.G.; Schertzer, J.D. Glucose alters the symbiotic relationships between gut microbiota and host physiology. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E111–E116.
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.-C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103.
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 2015, 31, 69–75.
- Ticinesi, A.; Lauretani, F.; Milani, C.; Nouvenne, A.; Tana, C.; Del Rio, D.; Maggio, M.; Ventura, M.; Meschi, T. Aging Gut Microbiota at the Cross-Road between Nutrition, Physical Frailty, and Sarcopenia: Is There a Gut-Muscle Axis? Nutrients 2017, 9, 1303.
- Ragonnaud, E.; Biragyn, A. Gut microbiota as the key controllers of “healthy” aging of elderly people. Immun. Ageing 2021, 18, 2.
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.B.; Wood-Martin, R.; et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 2014, 63, 1913–1920.
- Schroeder, B.O.; Bäckhed, F. Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 2016, 22, 1079–1089.
- Qiu, Y.; Yu, J.; Li, Y.; Yang, F.; Yu, H.; Xue, M.; Zhang, F.; Jiang, X.; Ji, X.; Bao, Z. Depletion of gut microbiota induces skeletal muscle atrophy by FXR-FGF15/19 signalling. Ann. Med. 2021, 53, 508–522.
- Okamoto, T.; Morino, K.; Ugi, S.; Nakagawa, F.; Lemecha, M.; Ida, S.; Ohashi, N.; Sato, D.; Fujita, Y.; Maegawa, H. Microbiome potentiates endurance exercise through intestinal acetate production. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E956–E966.
- Suriano, F.; Van Hul, M.; Cani, P.D. Gut microbiota and regulation of myokine-adipokine function. Curr. Opin. Pharmacol. 2020, 52, 9–17.
- Gkekas, N.K.; Anagnostis, P.; Paraschou, V.; Stamiris, D.; Dellis, S.; Kenanidis, E.; Potoupnis, M.; Tsiridis, E.; Goulis, D.G. The effect of vitamin D plus protein supplementation on sarcopenia: A systematic review and meta-analysis of randomized controlled trials. Maturitas 2021, 145, 56–63.
- Tajiri, K.; Shimizu, Y. Branched-chain amino acids in liver diseases. Transl. Gastroenterol. Hepatol. 2018, 3, 47.
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376–381.
- Yoneshiro, T.; Wang, Q.; Tajima, K.; Matsushita, M.; Maki, H.; Igarashi, K.; Dai, Z.; White, P.J.; McGarrah, R.W.; Ilkayeva, O.R.; et al. BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature 2019, 572, 614–619.
- Kamei, Y.; Hatazawa, Y.; Uchitomi, R.; Yoshimura, R.; Miura, S. Regulation of Skeletal Muscle Function by Amino Acids. Nutrients 2020, 12, 261.
- Ticinesi, A.; Mancabelli, L.; Tagliaferri, S.; Nouvenne, A.; Milani, C.; Del Rio, D.; Lauretani, F.; Maggio, M.G.; Ventura, M.; Meschi, T. The Gut-Muscle Axis in Older Subjects with Low Muscle Mass and Performance: A Proof of Concept Study Exploring Fecal Microbiota Composition and Function with Shotgun Metagenomics Sequencing. Int. J. Mol. Sci. 2020, 21, 8946.
- Jeong, D.Y.; Ryu, M.S.; Yang, H.J.; Park, S. γ-PGA-Rich Chungkookjang, Short-Term Fermented Soybeans: Prevents Memory Impairment by Modulating Brain Insulin Sensitivity, Neuro-Inflammation, and the Gut-Microbiome-Brain Axis. Foods 2021, 10, 221.
- Chen, Y.; Zhou, J.; Wang, L. Role and Mechanism of Gut Microbiota in Human Disease. Front. Cell. Infect. Microbiol. 2021, 11, 625913.
- Ticinesi, A.; Nouvenne, A.; Cerundolo, N.; Catania, P.; Prati, B.; Tana, C.; Meschi, T. Gut Microbiota, Muscle Mass and Function in Aging: A Focus on Physical Frailty and Sarcopenia. Nutrients 2019, 11, 1633.
- Cruz-Jentoft, A.J.; Dawson Hughes, B.; Scott, D.; Sanders, K.M.; Rizzoli, R. Nutritional strategies for maintaining muscle mass and strength from middle age to later life: A narrative review. Maturitas 2020, 132, 57–64.
