Interest in the structure, function, and evolutionary relations of circulating and intracellular globins dates back more than 60 years to the first determination of the three-dimensional structure of these proteins. Non-erythrocytic globins have been implicated in circulatory control through reactions that couple nitric oxide (NO) signaling with cellular oxygen availability and redox status. Small artery endothelial cells (ECs) express free α-globin, which causes vasoconstriction by degrading NO. This reaction converts reduced (Fe2+) α-globin to the oxidized (Fe3+) form, which is unstable, cytotoxic, and unable to degrade NO. Therefore, (Fe3+) α-globin must be stabilized and recycled to (Fe2+) α-globin to reinitiate the catalytic cycle. The molecular chaperone α-hemoglobin-stabilizing protein (AHSP) binds (Fe3+) α-globin to inhibit its degradation and facilitate its reduction. The mechanisms that reduce (Fe3+) α-globin in ECs are unknown, although endothelial nitric oxide synthase (eNOS) and cytochrome b5 reductase (CyB5R3) with cytochrome b5 type A (CyB5a) can reduce (Fe3+) α-globin in solution.
- α-globin
- endothelial nitric oxide synthase (eNOS)
- cytochrome
- redox system
- arteries
- blood pressure
1. Introduction
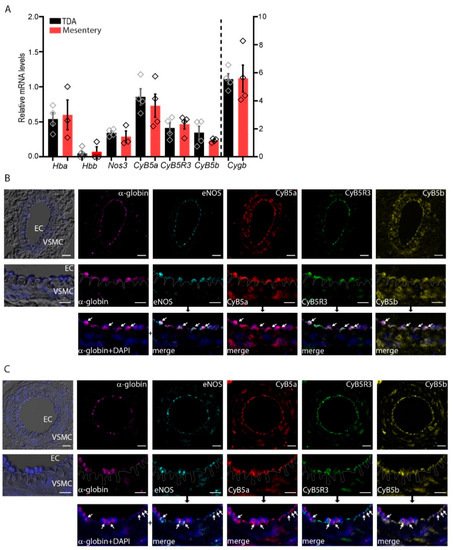
2. α-Globin, CyB5R3/CyB5a, and eNOS Are Expressed in Arterioles
3. eNOS Reductase and Oxygenase Domain Activities
| Enzymatic System | Reduction Rate (s−1) ± SD | Conditions | |
|---|---|---|---|
| α-Globin–AHSP (1 µM) |
CyB5R3/CyB5a/NADH (0.1 µM/1 µM/100 µM) |
0.003 ± 0.00004 | 76 Torr CO |
| CyB5R3/NADH (0.1 µM/100 µM) |
0 | 76 Torr CO/10 Torr O2 | |
| eNOS/NADPH/BH4/CaM/Ca2+ (1 µM/50 µM/50 µM/3 µM/1.4 mM) |
0.04 ± 0.0007 | 76 Torr CO | |
| eNOS/NADPH/BH4/CaM/Ca2+/L-arginine (1 µM/50 µM/50 µM/3 µM/1.4 mM/0.2 mM) |
0.05 ± 0.0019 | Anaerobic | |
| eNOS (1 µM) |
NADPH/BH4/CaM/Ca2+ (50 µM/50 µM/3 µM/1.4 mM) |
0.008± 0.00005 | 76 Torr CO |
| NADPH/BH4//CaM/Ca2+/L-arginine (50 µM/50 µM/3 µM/1.4 mM/0.2 mM) |
0.003 ± 0.00002 | Anaerobic |
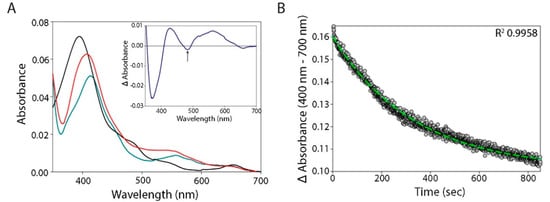
4. Reduction of α-Globin by eNOS
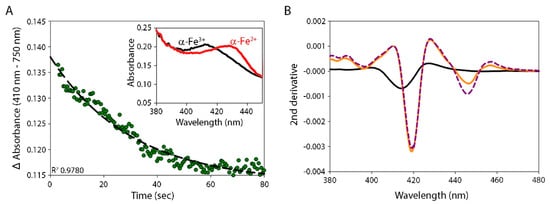
5. Reduction of α-Globin by the CyB5R3/CyB5a System
This entry is adapted from the peer-reviewed paper 10.3390/antiox11010159
References
- Freitas, T.A.K.; Hou, S.; Dioum, E.M.; Saito, J.A.; Newhouse, J.; Gonzalez, G.; Gilles-Gonzalez, M.-A.; Alam, M. Ancestral hemoglobins in Archaea. Proc. Natl. Acad. Sci. USA 2004, 101, 6675–6680.
- Vinogradov, S.N.; Hoogewijs, D.; Bailly, X.; Arredondo-Peter, R.; Gough, J.; Dewilde, S.; Moens, L.; Vanfleteren, J.R. A phylogenomic profile of globins. BMC Evol. Biol. 2006, 6, 31.
- Vinogradov, S.N.; Tinajero-Trejo, M.; Poole, R.K.; Hoogewijs, D. Bacterial and archaeal globins—A revised perspective. Biochim. Biophys. Acta 2013, 1834, 1789–1800.
- Hoppe, F. Ueber das Verhalten des Blutfarbstoffes im Spectrum des Sonnenlichtes. Virchows Arch. 1862, 23, 446–449.
- Gell, D.A. Structure and function of haemoglobins. Blood Cells Mol. Dis. 2018, 70, 13–42.
- Kiger, L.; Tilleman, L.; Geuens, E.; Hoogewijs, D.; Lechauve, C.; Moens, L.; Dewilde, S.; Marden, M.C. Electron transfer function versus oxygen delivery: A comparative study for several hexacoordinated globins across the animal kingdom. PLoS ONE 2011, 6, e20478.
- Burmester, T.; Hankeln, T. Function and evolution of vertebrate globins. Acta Physiol. 2014, 211, 501–514.
- Saha, D.; Patgaonkar, M.; Shroff, A.; Ayyar, K.; Bashir, T.; Reddy, K.V.R. Hemoglobin expression in nonerythroid cells: Novel or ubiquitous? Int. J. Inflam. 2014, 2014, 803237.
- Keller, T.C.S.; Lechauve, C.; Keller, A.S.; Brooks, S.; Weiss, M.J.; Columbus, L.; Ackerman, H.C.; Cortese-Krott, M.M.; Isakson, B.E. The role of globins in cardiovascular physiology. Physiol. Rev. 2021.
- Halligan, K.E.; Jourd’heuil, F.L.; Jourd’heuil, D. Cytoglobin is expressed in the vasculature and regulates cell respiration and proliferation via nitric oxide dioxygenation. J. Biol. Chem. 2009, 284, 8539–8547.
- Straub, A.C.; Lohman, A.W.; Billaud, M.; Johnstone, S.R.; Dwyer, S.T.; Lee, M.Y.; Schoppee Bortz, P.; Best, A.K.; Columbus, L.; Gaston, B.; et al. Endothelial cell expression of haemoglobin α regulates nitric oxide signalling. Nature 2012, 491, 473–477.
- Lechauve, C.; Butcher, J.T.; Freiwan, A.; Biwer, L.A.; Keith, J.M.; Good, M.E.; Ackerman, H.; Tillman, H.S.; Kiger, L.; Isakson, B.E.; et al. Endothelial cell α-globin and its molecular chaperone α-hemoglobin–stabilizing protein regulate arteriolar contractility. J. Clin. Investig. 2018, 128, 5073–5082.
- Liu, X.; El-Mahdy, M.A.; Boslett, J.; Varadharaj, S.; Hemann, C.; Abdelghany, T.M.; Ismail, R.S.; Little, S.C.; Zhou, D.; Thuy, L.T.T.; et al. Cytoglobin regulates blood pressure and vascular tone through nitric oxide metabolism in the vascular wall. Nat. Commun. 2017, 8, 14807.
- Shu, X.H.; Ruddiman, C.A.; Keller, T.C.S., 4th; Keller, A.S.; Yang, Y.; Good, M.E.; Best, A.K.; Columbus, L.; Isakson, B.E. Heterocellular contact can dictate arterial function. Circ. Res. 2019, 124, 1473–1481.
- Gardner, A.M.; Cook, M.R.; Gardner, P.R. Nitric-oxide dioxygenase function of human cytoglobin with cellular reductants and in rat hepatocytes. J. Biol. Chem. 2010, 285, 23850–23857.
- Doyle, M.P.; Hoekstra, J.W. Oxidation of nitrogen oxides by bound dioxygen in hemoproteins. J. Inorg. Biochem. 1981, 14, 351–358.
- Gladwin, M.T.; Lancaster, J.R., Jr.; Freeman, B.A.; Schechter, A.N. Nitric oxide’s reactions with hemoglobin: A view through the SNO-storm. Nat. Med. 2003, 9, 496–500.
- Denton, C.C.; Shah, P.; Suriany, S.; Liu, H.; Thuptimdang, W.; Sunwoo, J.; Chalacheva, P.; Veluswamy, S.; Kato, R.; Wood, J.C.; et al. Loss of alpha-globin genes in human subjects is associated with improved nitric oxide-mediated vascular perfusion. Am. J. Hematol. 2021, 96, 277–281.
- Morgado, M.; Cairrão, E.; Santos-Silva, A.J.; Verde, I. Cyclic nucleotide-dependent relaxation pathways in vascular smooth muscle. Cell. Mol. Life Sci. 2012, 69, 247–266.
- Huang, P.L.; Huang, Z.; Mashimo, H.; Bloch, K.D.; Moskowitz, M.A.; Bevan, J.A.; Fishman, M.C. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature 1995, 377, 239–242.
- Scotland, R.S.; Madhani, M.; Chauhan, S.; Moncada, S.; Andresen, J.; Nilsson, H.; Hobbs, A.J.; Ahluwalia, A. Investigation of vascular responses in endothelial nitric oxide synthase/cyclooxygenase-1 double-knockout mice: Key role for endothelium-derived hyperpolarizing factor in the regulation of blood pressure in vivo. Circulation 2005, 111, 796–803.
- Tejero, J.; Shiva, S.; Gladwin, M.T. Sources of vascular nitric oxide and reactive oxygen species and their regulation. Physiol. Rev. 2019, 99, 311–379.
- Mas, M. A close look at the endothelium: Its role in the regulation of vasomotor tone. Eur. Urol. Suppl. 2009, 8, 48–57.
- Angelo, M.; Hausladen, A.; Singel, D.J.; Stamler, J.S. Interactions of NO with hemoglobin: From microbes to man. Methods Enzymol. 2008, 436, 131–168.
- Passon, P.G.; Hultquist, D.E. Soluble cytochrome b5 reductase from human erythrocytes. Biochim. Biophys. Acta 1972, 275, 62–73.
- Passon, P.G.; Reed, D.W.; Hultquist, D.E. Soluble cytochrome b5 from human erythrocytes. Biochim. Biophys. Acta 1972, 275, 51–61.
- Feng, L.; Zhou, S.; Gu, L.; Gell, D.A.; Mackay, J.P.; Weiss, M.J.; Gow, A.J.; Shi, Y. Structure of oxidized α-haemoglobin bound to AHSP reveals a protective mechanism for haem. Nature 2005, 435, 697–701.
- Straub, A.C.; Butcher, J.T.; Billaud, M.; Mutchler, S.M.; Artamonov, M.V.; Nguyen, A.T.; Johnson, T.; Best, A.K.; Miller, M.P.; Palmer, L.A.; et al. Hemoglobin α/eNOS coupling at myoendothelial junctions is required for nitric oxide scavenging during vasoconstriction. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2594–2600.
- Amdahl, M.B.; Sparacino-Watkins, C.E.; Corti, P.; Gladwin, M.T.; Tejero, J. Efficient reduction of vertebrate cytoglobins by the cytochrome b5/cytochrome b5 reductase/NADH system. Biochemistry 2017, 56, 3993–4004.
- James, R.; Searcy, J.L.; Le Bihan, T.; Martin, S.F.; Gliddon, C.M.; Povey, J.; Deighton, R.F.; Kerr, L.E.; McCulloch, J.; Horsburgh, K. Proteomic analysis of mitochondria in APOE transgenic mice and in response to an ischemic challenge. J. Cereb. Blood Flow Metab. 2012, 32, 164–176.
- Abu-Soud, H.M.; Ichimori, K.; Presta, A.; Stuehr, D.J. Electron transfer, oxygen binding, and nitric oxide feedback inhibition in endothelial nitric-oxide synthase. J. Biol. Chem. 2000, 275, 17349–17357.
- Henderson, C.J.; McLaughlin, L.A.; Wolf, C.R. Evidence that cytochrome b5 and cytochrome b5 reductase can act as sole electron donors to the hepatic cytochrome P450 system. Mol. Pharmacol. 2013, 83, 1209–1217.
- Gacon, G.; Lostanlen, D.; Labie, D.; Kaplan, J.C. Interaction between cytochrome b5 and hemoglobin: Involvement of β66 (E10) and β95 (FG2) lysyl residues of hemoglobin. Proc. Natl. Acad. Sci. USA 1980, 77, 1917–1921.
- Wheeler, K.E.; Nocek, J.M.; Cull, D.A.; Yatsunyk, L.A.; Rosenzweig, A.C.; Hoffman, B.M. Dynamic docking of cytochrome b5 with myoglobin and α-hemoglobin: Heme-neutralization “squares” and the binding of electron-transfer-reactive configurations. J. Am. Chem. Soc. 2007, 129, 3906–3917.
