Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Agriculture, Dairy & Animal Science
Miniaturization advances have led to several wearable sensors that are now being employed in a variety of biomedical applications. Some of these have been ingrained in people’s daily lives. Smart bands and smartwatches with pulse monitors, pulse oximeters, accelerometers, gyroscopes, and other sensors are one example.
- wearable sensors
- skin-like
- heart rate monitoring
- continuous glucose monitoring
1. Introduction
Miniaturization advances have led to several wearable sensors that are now being employed in a variety of biomedical applications [1][2]. Some of these have been ingrained in people’s daily lives [3]. Smart bands and smart watches with pulse monitors, pulse oximeters, accelerometers, gyroscopes, and other sensors are one example [4][5][6]. Implantable devices have been utilized since the 1950s when the first pacemaker was utilized to bring back a regular heart rhythm by continually pumping only the ventricle [7]; since then, implantable automation has evolved to include the most modern implants, for instance Ocular and Cochlear implants [8][9]. Even though biomedical implants are vital to the advancement of Medicare, they frequently necessitate the assistance of a trained specialist [10]. Wearables, on the other hand, are more intelligible and may be employed without the need for medical or technical competence [11]. Their purpose is to harvest data without requiring any surgical processes or the insertion of materials that are more likely to cause long-term negative effects. These sensors can be worn anywhere one wants to wear them. While this sounds like an exaggeration, with sensors implanted in clothing and other form factors in addition to wrist-worn devices, it is principally true. Figure 1 shows the rise of wearables and the future of wearable technology [12].
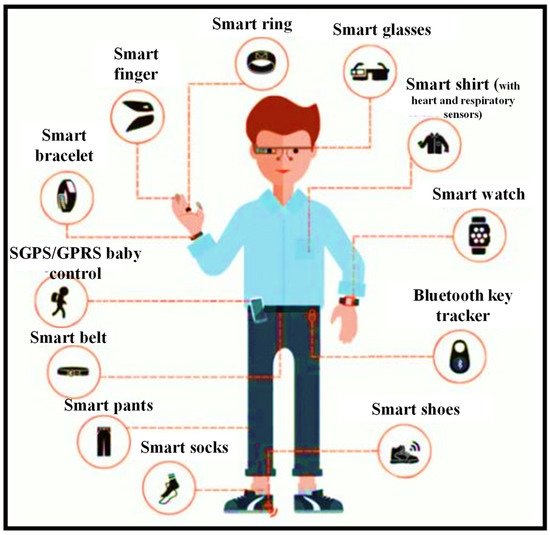
Figure 1. Wearable sensors can be worn almost anywhere on the body for daily activities, including health monitoring [12].
The application of wearable devices and arrangements to accomplish immediate recognition of variations in patient status necessitating therapeutic intervention is a rising topic of study in the field of wearables. The care of patients with chronic obstructive pulmonary disease (COPD) is one instance of this sort of wearable use. Early diagnosis of exacerbation events is an important objective in the therapeutic care of patients with COPD. Exacerbations, which are described as bouts of augmented dyspnea, cough, and a variation in the volume and type of sputum, are a regular occurrence in the natural course of COPD, and they can cause functional impairments and disability. Exacerbations should be detected and treated as soon as possible to avoid worsening clinical conditions and the need for emergency department care or hospital admission. Remote monitoring devices can aid in the early detection of patterns in patient’s health state that indicate an impending exacerbation. One way to address the problem of early identification of exacerbation episodes is to monitor variations in patient’s level of activity and presume that a drop in activity level indicates the possibility of a deterioration of the individual’s clinical state without further monitoring [13][14].
Atallah et al. have created an ear-worn sensor that may be employed to track activities and degrees of effort in patients with COPD [15]. The researchers were able to recognize numerous different types of physical activities, as well as the intensity of those activities, using powerful machine learning techniques and a single ear-worn sensor. Steele et al. [16] and Belze et al. [17] measured human movement in 3D over three days and found that the extent of the acceleration vector verified in patients with COPD was correlated with measures of patient status, such as the six-minute walk distance, the FEV1 (Force Expiratory Volume in One Second), the severity of dyspnea, and the physical function domain of the health-related quality of life scale. Hecht et al. [18] proposed an algorithm established on data gathered using a single unit for a minute-by-minute study of patients’ activity levels. The method was put to a test in 22 patients for 14 days. The scientists also employed a simple empirically created algorithm to assess whether the person was wearing the gadget, allowing them to keep track of compliance. Another noteworthy finding from the same study was that during the first few days of assessment, individuals tended to increase their activity level. This finding shows that, monitoring should be performed regularly to prevent noticing the transient effects caused by the fact that the subject is aware that he or she is being watched.
Wearable sensing automation has quickly evolved from a science fantasy concept to a wide range of well-established user and medical devices [19]. The affordability and user-friendly arrangement offered by developments in miniaturized electronics, the development of smartphones and connected devices, an increasing consumer wish for health awareness, and the unmet prerequisite for medical practitioners to uninterruptedly acquire medical quality data from their patients are all contributing to the expansion of wearable sensors [20]. Despite initial success, there is still a thirst for even more data from the body. Most of the sensing modalities, including in current wearables (pulse, galvanic skin response, etc.) are non-specific, hence this desire remains unmet (e.g., how many factors can increase one’s pulse or trigger one to sweat). Additionally, most wearable sensor devices use techniques that have been around for years. Even the most complex wearables, such as continuous transdermal glucose monitors, benefit from over 30 years of advancements in enzyme electrodes discovered in basic and ultra-low-cost finger-prick glucose test strips. Transdermal glucose assessment is, in fact, possibly the only widely employed wearable sensor that continuously monitors the state of a serious condition (diabetes) [21][22].
Figure 2 is a conceptual picture of a remote surveillance system [19]. Wearable sensors collect physiological and movement data, allowing patients’ status to be monitored. Sensors are employed in different ways depending on the therapeutic use of interest. When monitoring patients with congestive heart failure or COPD who are receiving clinical intervention, sensors for monitoring vital signs would be deployed. Sensors for recording movement data might be employed in applications monitoring the efficacy of home-based recovery programs in stroke survivors or in the usage of mobility assistance equipment among the elderly. Patients’ data are transmitted to a mobile phone or an access point through wireless transmission that is then relayed to a distant center over the internet. Data processing applied throughout the arrangement detects emergency circumstances, and an alert message is delivered to a trauma service center to give rapid aid to patients. Family members and caregivers are contacted in the event of an emergency, but they may also be informed in other instances, such as when the patient needs help taking his/her prescriptions. Clinical staff can remotely examine the patient’s condition and be notified if a medical decision must be taken.
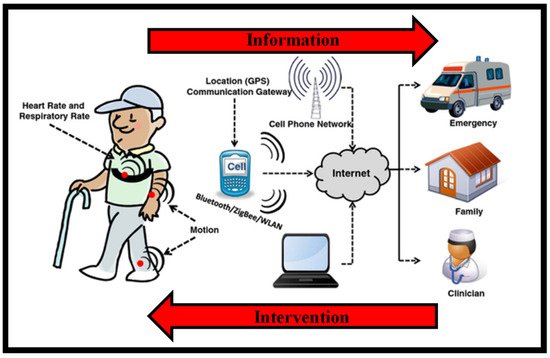
Figure 2. Design of a remote health observing arrangement established on wearables. Health-related data are collected via body-worn wireless devices and transferred to the caregiver via a data gateway, for instance, a mobile phone. Caregivers can utilize this data to execute interventions as required [19].
Almost every analyte that a clinician could want to assess from a patient can now be measured using diagnostic instruments [23][24][25]. Unfortunately, such devices are not wearable, and blood draws and traditional bench-top analysis procedures are still required. Therefore, the key issue on many people’s minds is: how can wearable sensor automation begin to cross over into modalities that detect more precise physiological issues, such as: validating a child’s health while in the mother’s womb by tracking mechanical fetal motion; telling the difference between a deadly seizure and greater physical exertion; warning that an athlete or worker is becoming severely dehydrated; informing the health-conscious about how much overly refined white bread boosted their blood sugar levels; or mapping and controlling viral infection in a community before most of the population becomes symptomatic.
2. Demand for Wearable Optical Sensors
Even though both electronics and photonics are important in the prospect of wearables, this paper will concentrate on wearable optical devices. Optical sensors are expected to account for 13% of the wearable market by 2020, with optical and optoelectronic (OE) technologies also playing a role in other market segments, for instance chemical or elastic and pressure sensors. In recent years, several platforms have been used to produce optical sensors for refractive index sensing applications [26][27][28][29][30]. Optical sensors are distinct in that they are resistant to electromagnetic radiation, can probe nanoscale volumes, permit unintrusive examination of biological substance at comparatively deep penetration depths, and frequently use low-cost, water-, and corrosion-resistant sensing components. These resources have been used to detect and quantify ion, protein, and viral concentration, as well as pulse, blood pressure (BP), blood oxygenation, abdominal and thoracic respiration rate, targeted localized bending, and movement. Optical sensors, like all other sensing devices, must handle the magnified problems of proper signal-to-noise ratio (SNR), restricted dynamic range, signal specificity, and user variability in the setting of wearable devices. Furthermore, there is the issue of surrounding light interfering with signal readings, as well as poor light penetration into the skin and other bio-fluids, which is unique to optical sensing devices. New optical sensing elements and integration techniques, such as photonic textiles [31], innovative colorimetric [32] and fluorometric materials [33], and flexile photonics [34], are currently being researched to solve these difficulties.
By 2025, the worldwide wearable sensing industry is expected to be worth USD 5.5 billion, with currently developing technologies accounting for nearly a third of that total [35]. Over 1/10 Americans now possess a wearable sensing gadget, such as a specialized fitness surveillance device, a threefold increase from 2012 [36]. Fitness trackers and smartwatches can create personalized health profiles by gathering data on the pulse, blood oxygen level, movement, speed, step count, and even eating and sleeping patterns, using mobile phones and cloud connections [37]. Such gadgets are especially appealing for at-home health surveillance, particularly for the rising number of seniors who are living independently. Wearables can offer a reliable and thorough patient health record, minimize the resource load on hospitals, and expedite the reaction time in event of an emergency by empowering older users, their families, caregivers, and Medicare professionals, through remote health surveillance capabilities. Wearable technologies for elderly health surveillance are already making an effect, with total device shipments connected to wearable technologies for elderly health surveillance expected to achieve USD 44 million in 2019. Wearables are also becoming the latest means for medical practitioners and healthcare workers for hand-free, computationally assisted quick diagnoses and other health decision making, through ergonomic displays and voice control features. Wearables may also be employed to create augmented reality, for reasons such as better viewing of vital organs and tissue during surgery [38]. Wearable sensing device automation is even being employed for environmental surveillance. The capacity to simply monitor plant health, air quality, or toxins across a vast region via crowdsourcing is interesting, and certain new wearable devices make it even easier. Wearable sensing devices have been researched around the world. Figure 3 shows the number of publications indexed in the Scopus database published in the last four decades (1982–2022) concerning the countries. It can be seen that the USA is leading this research topic with the highest number of publications, which is two times higher than the rest of the world (R.O.T.W).
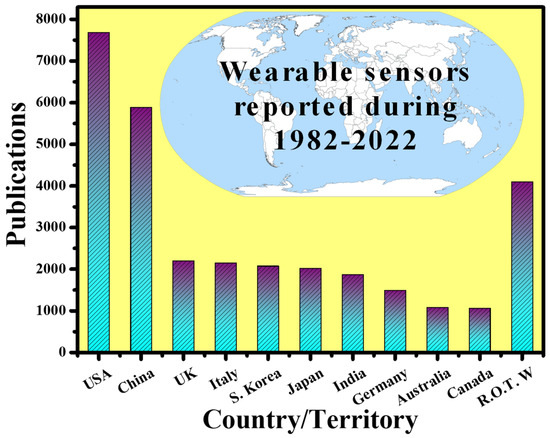
Figure 3. Scientific publications on “wearable sensors (electronics and photonics)” around the world. Results were collected on 19 November 2021.
Recent advancements in manufacturing and packaging processes have supported the low-cost embedding of many OE devices and sensing devices on a chip. Furthermore, these chips are extensively employed in many kinds of physiological, biomechanical, and biological sensing, examining and gathering biomedical data remotely, thanks to a combination of wireless 3G and 4G technologies [39]. This has resulted in lower Medicare expenditure and enhanced continuous tracking of critical data, particularly for athletes, resulting in increased performance [2]. The development of 5G networks, paired with technologies such as the internet of things (IoT), machine learning, and artificial intelligence, will further revolutionize the future of remote biomedical sensing. Today, medical practitioners use portable diagnostic instruments, such as glucometers, which offer instantaneous data and are typically unintrusive or less intrusive [40].
However, automation for totally unintrusive blood sugar tracking is currently being developed. As a result, the main goal here is to build unintrusive wearable sensing devices for these sorts of diagnostics that may be employed in uninterrupted tracking procedures. Furthermore, the population is rapidly growing, and physicians are in limited supply all around the world. This has compelled the ordinary person to look for alternate choices that would help physicians use their time more efficiently. Individuals can use wearables to trace their important physiological data, with the option to visit a medical practitioner only when necessary [41]. The growing need for personal diagnosis and tracking is a robust sign of the benefits of wearables. Their application is not limited to tracking blood sugar levels; they have recently been anticipated as a substitute scheme for performing rapid HIV diagnoses [42][43], timely recognition of Alzheimer’s syndrome [44][45], and perspiration tracking via a wearable paper-based sweat sensing device [46], taking custom-made medicine to a new level [47]. Wearable interfaces, such as wearable electronics, electronic skin sensing devices, flexile displays, intelligent robotics, and implanted medical devices, have advanced rapidly in recent years [48]. Due to their unique structure, most of these devices are flexile, portable, adaptable, and easy to use, and they can even be directly bonded with human skin [37][49][50].
There are several non-implantable wearable sensors available for consumer health and medical research, and some devices are currently being used in regular clinical practice. Table 1 lists some of the most prevalent devices [51]. Mechanical, physiological, and biochemical sensors are the three basic types of sensors available. Sensors are available in a variety of grades, from consumer to clinical to research grade. The data from these sensors have been utilized for a variety of purposes, including tracking gait, diagnosing atrial fibrillation, and measuring blood glucose, to name a few.
Table 1. Examples of common consumer, clinical, and research-grade wearable sensors [51].
| Manufacturer | Model | Market | Cost (USD) | Form Factor | Sensors | US FDA Status | Ref. |
|---|---|---|---|---|---|---|---|
| Abbott | Libre | Ambulatory diabetes monitoring | 149.98 (cost for reader and 10-day sensor) | Semi-invasive | CGM | Approved | [52][53] |
| AliveCor | Kardia Band | Consumer | 199 | Wristband | ECG | Cleared | [54] |
| Apple | Watch Series 3 | Consumer | 329 | Watch | Accel, ambient light sensor, BALT, Gyro, PPG HR, GPS | Pre-certified | [55] |
| Ava Science, Inc | Ava Wristband | Consumer | 249 | Wristband | Accel, EDA, PPG HR, Temperature sensors | Approved | [56] |
| Bloomlife | Smart Pregnancy tracker | Consumer (rental) | 20/week | Abdominal patch | Accel, 3-channel AFE | - | [56] |
| Preventice | Bodyguardian Heart | Ambulatory cardiac monitoring | Ordered through physician, billed directly to insurance | Chest patch | Accel, EFG | Cleared | [57] |
| Oura | Oura ring | Consumer | 299–999 | Ring | Accel, Gyro, PPG HR, Skin temperature | - | [58] |
| Orpyx | Surro Gait Rx | Ambulatory gait monitoring | Ordered through physician | Watch, shoe insert, shoe pod | Pressure | - | [59] |
| Orpyx | Surro Sense Rx | Ambulatory gait monitoring | Ordered through physician | Watch, shoe insert, shoe pod | Pressure | Cleared | [60] |
| iRhythm | Ziopatch | Ambulatory cardiac monitoring | Ordered through physician, billed directly to insurance | Chest patch | ECG | Cleared | [61] |
| Medtronic | Enlite | Ambulatory diabetes monitoring | - | Semi-invasive | CGM | Approved | [62] |
This entry is adapted from the peer-reviewed paper 10.3390/nano12030334
References
- Tricoli, A.; Nasiri, N.; De, S. Wearable and miniturized sensor technologies for personalized and preventive medicine. Adv. Funct. Mater. 2017, 27, 1605271.
- Koymedir, H.; Ozcan, A. Wearable and implantable sensors for biomedical applications. Annu. Rev. Anal. Chem. 2018, 11, 127–146.
- Kenry; Yeo, J.C.; Lim, C.T. Emerging flexible and wearable physical sensing platforms for healthcare and biomedical applications. Microsyst. Nanoeng. 2016, 2, 16043.
- Auepanwiriyakul, C.; Waibel, S.; Songa, J.; Bentley, P.; Faisal, A.A. Accuracy and Acceptability of Wearable Motion Tracking for Inpatient Monitoring Using Smartwatches. Sensors 2020, 20, 7313.
- Fu, Y.; Liu, J. System Design for Wearable Blood Oxygen Saturation and Pulse Measurement Device. Procedia Manuf. 2015, 3, 1187–1194.
- Casilari, E.; Álvarez-Marco, M.; García-Lagos, F. A Study of the Use of Gyroscope Measurements in Wearable Fall Detection Systems. Symmetry 2020, 12, 649.
- Lim, G.B. Pacemaker powered by cardiac motion. Nat. Rev. Cardiol. 2019, 16, 386.
- Haghjou, N.; Soheilian, M.; Abdekhodaie, M.J. Sustained Release Intraocular Drug Delivery Devices for Treatment of Uveitis. J. Ophthalmic Vis. Res. 2011, 6, 317–329.
- Lo, R.; Li, P.-Y.; Saati, S.; Agrawal, R.N.; Humayun, M.S.; Meng, E. A passive MEMS drug delivery pump for treatment of ocular diseases. Biomed. Microdevices 2009, 11, 959–970.
- Manivasagam, G.; Dhinasekaran, D.; Rajamanickam, A. Biomedical implants: Corrosion and its prevention—A review. Recent Pat. Corros. Sci. 2010, 2, 40–54.
- Msc, M.J.M.B.; Huizinga, E.; Van Loon, K.; Leenen, L.P.H.; Dohmen, D.A.J.; Kalkman, C.J.; Blokhuis, T.J. Reliability of wireless monitoring using a wearable patch sensor in high-risk surgical patients at a step-down unit in the Netherlands: A clinical validation study. BMJ Open 2018, 8, e020162.
- Available online: https://www.sensortips.com/featured/where-are-wearable-sensors-worn/ (accessed on 1 December 2021).
- Moy, M.L.; Mentzer, S.J.; Reilly, J.J. Ambulatory monitoring of cumulative free-living activity. IEEE Eng. Med. Boil. Mag. 2003, 22, 89–95.
- Sherrill, D.M.; Moy, M.L.; Reilly, J.J.; Bonato, P. Using hierarchical clustering methods to classify motor activities of COPD patients from wearable sensor data. J. Neuroeng. Rehabil. 2005, 2, 16.
- Atallah, L.; Zhang, J.; Lo, B.P.; Shrikrishna, D.; Kelly, J.L.; Jackson, A.; Polkey, M.I.; Yang, G.-Z.; Hopkinson, N.S. Validation of an Ear Worn Sensor for Activity Monitoring in COPD. Am. J. Respir. Crit. Care Med. 2010, 181, A1211.
- Steele, B.G.; Holt, L.; Belza, B.; Ferris, S.; Lakshminaryan, S.; Buchner, D.M. Quantitating Physical Activity in COPD Using a Triaxial Accelerometer. Chest 2000, 117, 1359–1367.
- Belza, B.; Steele, B.G.; Hunziker, J.; Lakshminaryan, S.; Holt, L.; Buchner, D.M. Correlates of Physical Activity in Chronic Obstructive Pulmonary Disease. Nurs. Res. 2001, 50, 195–202.
- Hecht, A.; Ma, S.; Porszasz, J.; Casaburi, R.; for the COPD Clinical Research Network. Methodology for Using Long-Term Accelerometry Monitoring to Describe Daily Activity Patterns in COPD. COPD J. Chronic Obstr. Pulm. Dis. 2009, 6, 121–129.
- Patel, S.; Park, H.; Bonato, P.; Chan, L.; Rodgers, M. A review of wearable sensors and systems with application in rehabilitation. J. Neuroeng. Rehabil. 2012, 9, 21.
- Nachiar, C.C.; Ambika, N.; Moulika, R.; Poovendran, R. Design of Cost-effective Wearable Sensors with integrated Health Monitoring System. In Proceedings of the Fourth International Conference on I-SMAC (IoT in Social, Mobile, Analytics and Cloud) (I-SMAC), Palladam, India, 7–9 October 2020; pp. 1289–1292.
- Cappon, G.; Acciaroli, G.; Vettoretti, M.; Facchinetti, A.; Sparacino, G. Wearable Continuous Glucose Monitoring Sensors: A Revolution in Diabetes Treatment. Electronics 2017, 6, 65.
- Jin, X.; Li, G.; Xu, T.; Su, L.; Yan, D.; Zhang, X. Fully integrated flexible biosensor for wearable continuous glucose monitoring. Biosens. Bioelectron. 2021, 196, 113760.
- Butt, M.; Khonina, S.; Kazanskiy, N. Plasmonics: A Necessity in the Field of Sensing—A Review (Invited). Fiber Integr. Opt. 2021, 40, 14–47.
- Butt, M.A.; Khonina, S.N.; Kazanskiy, N.L. Plasmonic refractive index sensor based on metal–insulator-metal waveguides with high sensitivity. J. Mod. Opt. 2019, 66, 1038–1043.
- Butt, M.A.; Khonina, S.N.; Kazanskiy, N.L. Silicon on silicon dioxide slot waveguide evanescent field gas absorption sensor. J. Mod. Opt. 2017, 65, 174–178.
- Butt, M.A. Numerical investigation of a small footprint plasmonic Bragg grating structure with a high extinction ratio. Photonics Lett. Pol. 2020, 12, 82–84.
- Butt, M.A.; Khonina, S.N.; Kazanskiy, N.L. Sensitivity Enhancement of Silicon Strip Waveguide Ring Resonator by Incorporating a Thin Metal Film. IEEE Sens. J. 2020, 20, 1355–1362.
- Butt, M.A.; Khonina, S.N.; Kazanskiy, N.L. Highly sensitive refractive index sensor based on hybrid plasmonic waveguide microring resonator. Waves Random Complex Media 2020, 30, 292–299.
- Khonina, S.N.; Kazanskiy, N.L.; Butt, M.A. Evanescent Field Ratio Enhancement of a Modified Ridge Waveguide Structure for Methane Gas Sensing Application. IEEE Sens. J. 2020, 20, 8469–8476.
- Butt, M.A.; Kazanskiy, N.L.; Khonina, S.N. Highly Sensitive Refractive Index Sensor Based on Plasmonic Bow Tie Configuration. Photonics Sens. 2020, 10, 223–232.
- Leal-Junior, A.; Avellar, L.; Frizera, A.; Marques, C. Smart textiles for multimodal wearable sensing using highly stretchable multiplexed optical fiber system. Sci. Rep. 2020, 10, 13867.
- Nguyen, T.-H.; Mugherli, L.; Rivron, C.; Tran-Thi, T.-H. Innovative colorimetric sensors for the selective detection of monochloramine in air and in water. Sens. Actuators B Chem. 2015, 208, 622–627.
- Beutler, M.; Wiltshire, K.H.; Meyer, B.; Moldaenke, C.; Lüring, C.; Meyerhöfer, M.; Hansen, U.-P.; Dau, H. A fluorometric method for the differentiation of algal populations in vivo and in situ. Photosynth. Res. 2002, 72, 39–53.
- Righini, G.C.; Krzak, J.; Lukowiak, A.; Macrelli, G.; Varas, S.; Ferrari, M. From flexible electronics to flexible photonics: A brief overview. Opt. Mater. 2021, 115, 111011.
- Available online: Marketresearch.com (accessed on 1 December 2021).
- Tang, S.L.P. Wearable sensors for sports performance. Text. Sportsw. 2015, 169–196.
- Seshadri, D.R.; Li, R.T.; Voos, J.E.; Rowbottom, J.R.; Alfes, C.M.; Zorman, C.A.; Drummond, C.K. Wearable sensors for monitoring the physiological and biochemical profile of the athlete. NPJ Digit. Med. 2019, 2, 72.
- Kwasnicki, R.M.; Chen, C.-M.; Noakes, A.J.; Hettiaratchy, S.; Yang, G.-Z.; Darzi, A. Developing a Wearable Sensor for Continuous Tissue Oxygenation Monitoring: A Proof of Concept Study. J. Reconstr. Microsurg. Open 2021, 6, e11–e19.
- Majumder, S.; Mondal, T.; Deen, M.J. A Simple, Low-Cost and Efficient Gait Analyzer for Wearable Healthcare Applications. IEEE Sens. J. 2019, 19, 2320–2329.
- Johnston, L.; Wang, G.; Hu, K.; Qian, C.; Liu, G. Advances in Biosensors for Continuous Glucose Monitoring Towards Wearables. Front. Bioeng. Biotechnol. 2021, 9, 733810.
- Darwish, A.; Hassanien, A.E. Wearable and Implantable Wireless Sensor Network Solutions for Healthcare Monitoring. Sensors 2011, 11, 5561–5595.
- Kong, M.; Li, Z.; Wu, J.; Hu, J.; Sheng, Y.; Wu, D.; Lin, Y.; Li, M.; Wang, X.; Wang, S. A wearable microfluidic device for rapid detection of HIV-1 DNA using recombinase polymerase amplification. Talanta 2019, 205, 120155.
- Marquard, J.; Saver, B.; Kandaswamy, S.; Martinez, V.; Simoni, J.; Stekler, J.; Ganesan, D.; Scanlan, J. Designing a wrist-worn sensor to improve medication adherence: Accomodating diverse user behaviors and technology preferences. JAMIA Open 2018, 1, 153–158.
- Varatharajan, R.; Manogaran, G.; Priyan, M.K.; Sundarasekar, R. Wearable sensor devices for early detection of Alzheimer disease using dynamic time warping algorithm. Clust. Comput. 2018, 21, 681–690.
- Stavropoulos, T.G.; Lazarou, I.; Diaz, A.; Gove, D.; Georges, J.; Manyakov, N.V.; Pich, E.M.; Hinds, C.; Tsolaki, M.; Nikolopoulos, S.; et al. Wearable devices for assessing function in alzheimer’s disease: A european public involvement activity about the features and preferences of patients and caregivers. Front. Aging Neurosci. 2021, 13, 643135.
- Parrilla, M.; Guinovart, T.; Ferré, J.; Blondeau, P.; Andrade, F.J. A Wearable Paper-Based Sweat Sensor for Human Perspiration Monitoring. Adv. Health Mater. 2019, 8, 1900342.
- Cote, G.; Lec, R.; Pishko, M. Emerging biomedical sensing technologies and their applications. IEEE Sens. J. 2003, 3, 251–266.
- Wang, S.; Oh, J.Y.; Xu, J.; Tran, H.; Bao, Z. Skin-Inspired Electronics: An Emerging Paradigm. Acc. Chem. Res. 2018, 51, 1033–1045.
- Arif, M.; Kattan, A. Physical Activities Monitoring Using Wearable Acceleration Sensors Attached to the Body. PLoS ONE 2015, 10, e0130851.
- Seshadri, D.R.; Li, R.T.; Voos, J.E.; Rowbottom, J.R.; Alfes, C.M.; Zorman, C.A.; Drummond, C.K. Wearable sensors for monitoring the internal and external workload of the athlete. NPJ Digit. Med. 2019, 2, 71.
- Dunn, J.; Runge, R.; Snyder, M. Wearables and the medical revolution. Pers. Med. 2018, 15, 429–448.
- Boscari, F.; Galasso, S.; Acciaroli, G.; Facchinetti, A.; Marescotti, M.C.; Avogaro, A.; Bruttomesso, D. Head-to-head comparison of the accuracy of Abbott FreeStyle Libre and Dexcom G5 mobile. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 425–427.
- Massa, G.G.; Gys, I.; Eyndt, A.O.; Bevilacqua, E.; Wijnands, A.; Declercq, P.; Zeevaert, R. Evaluation of the FreeStyle® Libre Flash Glucose Monitoring System in Children and Adolescents with Type 1 Diabetes. Horm. Res. Paediatr. 2018, 89, 189–199.
- Halcox, J.P.; Wareham, K.; Cardew, A.; Gilmore, M.; Barry, J.P.; Phillips, C.; Gravenor, M.B. Assessment of remote heart rhythm sampling using the AliveCor heart monitor to screen for atrial fibrillation: The REHEARSE-AF study. Circulation 2017, 136, 1784–1794.
- Tison, G.; Sanchez, J.M.; Ballinger, B.; Singh, A.; Olgin, J.E.; Pletcher, M.J.; Vittinghoff, E.; Lee, E.S.; Fan, S.M.; Gladstone, R.A.; et al. Passive Detection of Atrial Fibrillation Using a Commercially Available Smartwatch. JAMA Cardiol. 2018, 3, 409–416.
- Shilaih, M.; Clerck, V.; Falco, L.; Kubler, F.; Leeners, B. Pulse rate measurement during sleep using wearable sensors, and its correlation with the menstrual cycle phases, a prospective observational study. Sci. Rep. 2017, 7, 1294.
- O’Connor, K.L.; Rowson, S.; Duma, S.M.; Broglio, S.P. Head-Impact–Measurement Devices: A Systematic Review. J. Athl. Train. 2017, 52, 206–227.
- Zambotti, M.; Rosas, L.; Colrain, I.; Baker, F. The sleep of the ring: Comparison of the OURA sleep tracker against Polysomnography. Behav. Sleep Med. 2019, 17, 124–136.
- Vienneau, J.; Bauman, J.; Nigg, S.; Nigg, B.; Jarvis, S. Investigating the effects of the SurroGait Rx device on postural stability, gait, and MSIS-29 outcomes in people with multiple sclerosis. Biomed. J. Sci. Tech. Res. 2018, 2, 2329.
- Schlachetzki, J.C.M.; Barth, J.; Marxreiter, F.; Gossler, J.; Kohl, Z.; Reinfelder, S.; Gassner, H.; Aminian, K.; Eskofier, B.M.; Winkler, J.; et al. Wearable sensors objectively measure gait parameters in Parkinson’s disease. PLoS ONE 2017, 12, e0183989.
- Solomon, M.D.; Yang, J.; Sung, S.H.; Livingston, M.L.; Sarlas, G.; Lenane, J.C.; Go, A.S. Incidence and timing of potentially high-risk arrhythmias detected through long term continuous ambulatory electrocardiographic monitoring. BMC Cardiovasc. Disord. 2016, 16, 35.
- Sinha, M.; McKeon, K.M.; Parker, S.; Goergen, L.G.; Zheng, H.; El-Khatib, F.H.; Russell, S.J. A Comparison of Time Delay in Three Continuous Glucose Monitors for Adolescents and Adults. J. Diabetes Sci. Technol. 2017, 11, 1132–1137.
This entry is offline, you can click here to edit this entry!
