Megalobrama, a genus of cyprinid fish, is an economically important freshwater fish widely distributed in major waters of China. Here, we report the genome resequencing of 180 Megalobrama fish including M. amblycephala, M. skolkovii, M. hoffmanni, and M. pellegrini. Population structure indicated that geographically divergent Megalobrama populations were separated into six subgroups. A phylogenetic tree showed that M. skolkovii was more closely related to M. pellegrini than other species and M. hoffmanni was clustered apart from other Megalobrama species, showing a high nucleotide diversity in geographic groups. Treemix validated gene flow from M. amblycephala to M. skolkovii, suggesting that introgression may provide an important source of genetic variation in the M. skolkovii populations. According to the demographic history analysis, it is speculated that Megalobrama might have been originally distributed in the Pearl River with some spread to Hainan Island and northern China due to lower sea levels during the glacial period. Whole-genome selective sweeps analysis demonstrated that M. amblycephala likely developed an enhanced energy metabolism mostly through fatty acid degradation pathways whereas M. hoffmanni possibly regulate lipid absorption via the cholesterol metabolism pathway. Taken together, this study provides a valuable genomic resource for future genetic investigations aiming to improve genome-assisted breeding of Megalobrama species.
1. Introduction
Megalobrama is a cyprinid fish genus that belongs to the subfamily Cultrinae (Cypriniformes, Cyprinidae). It is one of the economically most important fish species, as well as the main aquaculture species in China. The genus
Megalobrama contains four recognized species,
M. amblycephala,
M. skolkovii,
M. hoffmanni, and
M. pellegrini [
1]. Previous studies categorized the four species mainly based on morphologic traits and geographic distribution. For instance,
M.
amblycephala is distinguished by the morphology of the upper orbital bone and oral fissure, and also the caudal peduncle depth/length ratio [
2]. It is mainly distributed in large- and medium-sized lakes in the middle and lower branches of the Yangtze River [
3].
M. skolkovii differs from the other three species in the longer length of the first swim bladder [
2]. It inhabits the Amur, Yangtze, Yellow, and Minjiang rivers [
4].
M. hoffmanni has pigment deposits on the scale base that form dark spots on its body surface and exists mostly in the Pearl River and Hainan Island [
5].
M.
pellegrini dwells in the middle and upper reaches of the Yangtze River and has a unique upper jaw and upper orbital bone shape [
6]. Although previous studies have reported biological differences between
Megalobrama species, the phylogenetic relationships among
M. amblycephala,
M. skolkovii,
M. hoffmanni, and
M. pellegrini remain unknown [
7,
8].
Four
Megalobrama species have distinct feeding behaviors. For instance,
M. hoffmanni is an omnivorous fish that feeds mostly on benthic creatures such as freshwater shellfish, river clams, organic detritus, and some aquatic plants [
9].
M. skolkovii and
M. pellegrini are omnivorous as well, however, they primarily feed on aquatic plants [
10]. Importantly, in
M. pellegrini, the development of the upper and lower jaws as well as the thickening of the stratum corneum have strengthened the scraping function, making it more conducive to feeding on stationary organisms [
6].
M. amblycephala lives in aquatic plant-rich lakes and its diet consists primarily of aquatic plants, emergent plants, floating-leaf plants, and submerged plants. It is classified as herbivorous fish that mainly feeds on aquatic vascular plants [
11]. Obviously,
Megalobrama species must have evolved metabolic mechanisms that allow them to adapt to varying dietary compositions. However, it is not clear how
Megalobrama species regulate carbohydrate, lipid, and protein metabolism to maintain energy balance in the body.
Megalobrama germplasm resources have been rapidly depleting in recent years as a result of many factors, including environmental degradation factors. There are a few reports on
Megalobrama genetic resource conservation and population genetics [
1,
2,
4]. By using population genomics, the genetic information of species diversity can be established with the advent of high-throughput sequencing [
12,
13]. For instance, whole genome re-sequencing has been used to investigate the phylogenetic relationships among differentiated species [
14,
15]. Using the whole genome of
M. amblycephala as reference genome, this study was designed with the following objectives: (1) establish the whole genome database of
Megalobrama population using the whole genome re-sequencing technology; (2) explore the genetic structure of populations and infer detailed evolutionary relationships by phylogenetic tree, principal component analysis (PCA), and admixture analysis; (3) illustrate the demographic history of
Megalobrama species by pairwise sequentially Markovian coalescent (PSMC) method; (4) determine candidate genes related to variations in feeding habits utilizing selective sweeps analysis that would indicate the molecular mechanism (s) of environmental adaptability of
Megalobrama populations. This study will ascertain the genetic diversity, population history, and improve the understanding of the evolution of
Megalobrama populations and their molecular mechanisms of environmental adaptability. These findings will represent a significant contribution to conservation and utilization of
Megalobrama’s germplasm resources.
2. Genome Resequencing and Variation Calling
A total of 180
Megalobrama fish representing different geographical populations in China were selected for genome resequencing (
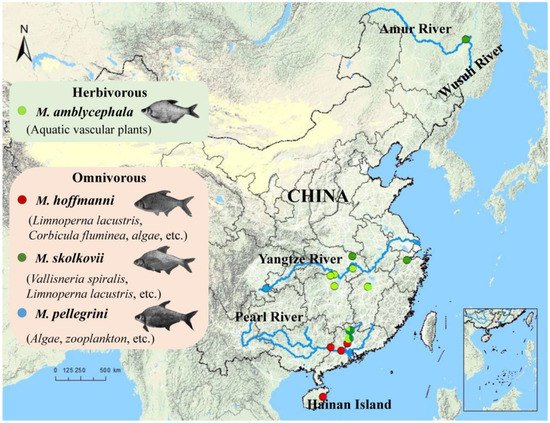
Figure 1,
Table S4). To explore the phylogenetic relationships and evolutionary history of
Megalobrama, all samples were sequenced to an average sequencing depth of ~17.43 × per individual yielding 94% sequencing coverage, an average Q20 of 97% and an average Q30 of 90% (
Table S5). After filtering and quality control, the sequencing generated a total of 4.063 Tb raw databases and 3.483 Tb clean databases. We mapped all individuals to the
M. amblycephala genome and detected 31,857,189 SNPs in the
Megalobrama populations (
Table S6).
Figure 1. Geographic map indicating the distribution and feeding habits of the Megalobrama species in this study. Circles reflect the geographic regions where the samples were collected from. The blue lines represent the rivers.
3. Phylogeny and Population Structure Analysis
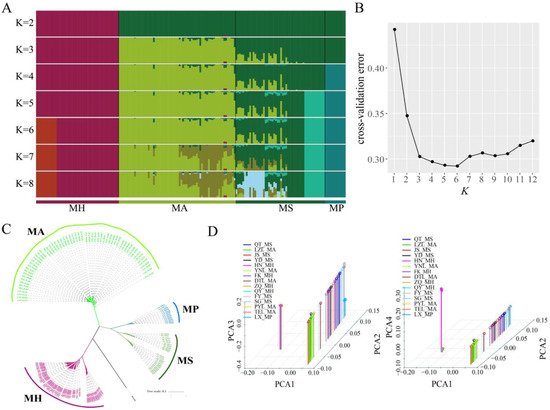
Admixture analysis was used to cluster individuals based on all high-confidence SNP sites. When ancestry components (K) = 6, six geographically distributed ancestral components (K) were labeled as: HN and Pearl River M. hoffmanni populations; M. amblycephala populations; FY and other M. skolkovii populations; and M. pellegrini populations (Figure 2A). This finding was supported by cross-validation (when K = 6, the cross-validation error was minimum) and was consistent with the phylogeny and biogeographic distribution (Figure 2B). Using the Danio rerio as an outgroup, a phylogenetic tree was constructed by the maximum likelihood method based on all non-admixed individuals. All of the samples were clustered into three branches. Initially, M. amblycephala and M. hoffmanni were clustered into a single branch and M. skolkovii and M. pellegrini clustered into one branch (Figure 2C). The findings revealed a close genetic affinity between M. skolkovii and M. pellegrini groups whereas the M. hoffmanni population showed genetic divergence from the other Megalobrama groups. Additionally, principal components analysis (PCA) provided supporting evidence for these groupings (Figure 2D). The first four principal components divided the Megalobrama populations into six subgroups. Taken together, the results provided compelling evidence that Megalobrama species from various geographical distributions can be divided into six different subgroups.
Figure 2. Phylogenetic analysis of different Megalobrama geographical populations. (A) Population genetic structure of 180 Megalobrama fish. The length of each colored segment represents the proportion of the individual genome inferred from ancestral populations (K = 2–8). The species name is at the bottom. (B) Cross-validation (CV) error for varying values of K in the admixture analysis. The minimum of estimated CV error on K = 6 suggests the most suitable number of ancestral populations. (C) Maximum-likelihood-based phylogenetic tree constructed using no admixed individuals. The scale bar represents pairwise distances between different individuals. Different colors represent different Megalobrama species. (D) Principal component analysis (PCA) of Megalobrama populations. Eigenvector 1, 2, 3, and 4 explained 45.06%, 18.03%, 4.00%, and 1.73% of the total variance, respectively. MH refers to M. hoffmanni, MA to M. amblycephala, MS to M. skolkovii, MP to M. pellegrini.
4. M. amblycephala Introgression into M. skolkovii
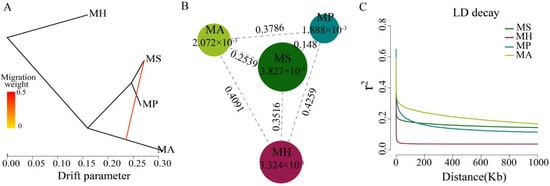
In this study, we used Treemix to confirm the gene flow from
M. amblycephala to
M. skolkovii. Using
M. hoffmanni as the outgroup, among the four
Megalobrama species, only one migration event was inferred from the
M. amblycephala to the
M. skolkovii with approximately 50.77% DNA gene flow from
M. amblycephala to
M. skolkovii (
Figure 3A). Moreover, the migration patterns of these two species’ geographic populations demonstrated that LZL, DTL, TEL, YNL, and PYL
M. amblycephala populations introgressed into the FY, SG, QT, and JS
M. skolkovii populations. Notably, the migration weight of
M. amblycephala introgressed into QT and JS populations was greater than that of the FY and SG populations (
Figure S2).
Figure 3. Population genetic analysis of Megalobrama species. (A) Gene flow analysis of Megalobrama species. Arrows indicate migration events that occur between populations. The heat map indicates migration weight. (B) Nucleotide polymorphism (π), and differentiation index (Fst) of the four Megalobrama species. The largest circle represents the large π value, and the longer line segment represents the large Fst value. MH refers to M. hoffmanni, MA to M. amblycephala, MS to M. skolkovii, MP to M. pellegrini. (C) Decay of linkage disequilibrium (LD) patterns for the four Megalobrama species inferred by the phylogenetic trees.
5. Linkage Disequilibrium and Genetic Diversity
The genetic diversity (π) of M. skolkovii and M. hoffmanni were estimated to be 3.827 × 10−3 and 3.324 × 10−3, respectively, which was relatively high in comparison to M. amblycephala (2.072 × 10−3) and M. pellegrini (1.888 × 10−3). The low genetic differentiation (Fst) between M. skolkovii and M. pellegrini (0.148), and the high Fst between M. hoffmanni and M. amblycephala (0.4091), M. skolkovii (0.3516), and M. pellegrini (0.4259) (Figure 3B) are consistent with the phylogeny analysis. Between the four Megalobrama species, linkage disequilibrium (LD) and correlation coefficient (r2) values were calculated. The decay of LD reached half the maximum average r2 at a distance of 24 Kb, 4 Kb, 1.8 Kb, and 0.2 Kb for M. amblycephala, M. pellegrini, M. skolkovii, and M. hoffmanni, respectively (Figure 3C). Therefore, M. skolkovii and M. hoffmanni displayed a faster LD decay rate than M. amblycephala and M. pellegrini.
6. Demographic History of Megalobarma Species and Species Delimitation
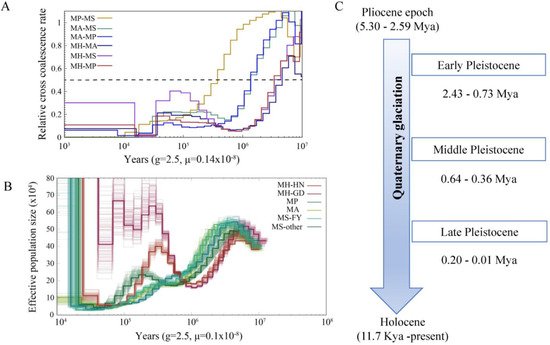
The split times based on the relative cross-coalescent rates (RCCR) among the four
Megalobrama species reached 0.5 suggesting a split between
M. hoffmanni and other species 3–5 Mya. The cross-coalescence analysis suggested a decline to 0.5 between
M. amblycephala and
M. skolkovii or
M. pellegrini at ~1.3 Mya, and a decline to 0.5 between
M. skolkovii and
M. pellegrini around 0.3–0.4 Mya (
Figure 4A). The PSMC method used to reconstruct the demographic history of six
Megalobrama subgroups indicated that the effective population size (
Ne) peak of
M. amblycephala and
M. pellegrini was 2.5 Mya and 4 Mya, respectively, compared to 3–4 Mya of the
Ne peak for the
M. skolkovii subgroups. After that, the FY
M. skolkovii subgroup continued to shrink, whilst the
Ne curves of other
M. skolkovii subgroup split at 0.2 Mya expanded (
Figure 4B). Moreover, the two
Ne peaks of
M. hoffmanni occurred at 0.3 Mya and 5 Mya, respectively, during the middle pleistocene geological period (
Figure 4C). Interestingly, the split of
Ne curves of the two
M. hoffmanni subgroups occurred about 0.4 Mya, indicating a population divergence at this time. The ancestral reconstruction analysis revealed two different biogeographic evolutionary processes to investigate the ancestral distribution of genus
Megalobrama. According to the BBM analysis, the genus
Megalobrama was originally distributed in the Pearl River and then spread to Hainan Island and Northern China. However, the analysis based on the S-DIVA and DEC models showed that the
Megalobrama ancestors originally inhabited the Pearl River and Yangtze River, before spreading to the Amur and Wusuli Rivers (
Figure S3).
Figure 4. Demographic history of Megalobrama species. (A) Relative cross coalescence rates (CCR) between Megalobrama populations. When the two populations are completely mixed, the CCR is close to one. When they are completely split, the CCR is close to zero. The dotted line indicates that the CCR is 0.5. MP, MS, MA, and MH refer to M. pellegrini, M. skolkovii, M. amblycephala, and M. hoffmanni. g (generation time) = 2.5 years; μ (neutral mutation rate per generation) = 0.14 × 10−8. (B) PSMC model estimates changes in the effective population size over time, representing variation in inferred Ne dynamics. The undulating broken line in the figure is the estimated effective population size of each population in the evolutionary history. The time axis is not divided into deciles. μ = 0.1 × 10−8. MH-HN, MH-GD, MP, MA, MS-FY, and MS-other refer to the Hainan population of M. hoffmanni, Pearl River populations of M. hoffmanni, M. pellegrini, M. amblycephala population, Fuyuan population of M. skolkovii, and other M. skolkovii populations. (C) Timeline of Quaternary glaciation. Mya = million years ago.
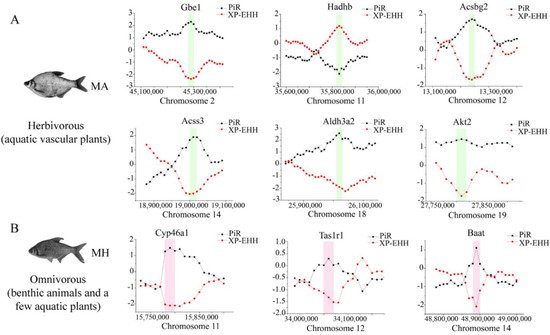
7. Selective Sweeps for Dietary Adaptation of Megalobrama
To investigate the potential selective signals during
M. amblycephala dietary adaptation, we scanned the genomic regions based on genome-wide calculations for selective sweeps by estimating Fst, PiR, and XP-EHH values. Candidate genes were discovered in the common region of top 5% Fst, High/Low top 5% PiR, and High/Low top 5% XP-EHH, inferred from the comparisons between
M. amblycephala and the other three species (
Figure 5A,
Figures S4 and S5A). Fatty acid degradation, glycerolipid metabolism, beta-alanine metabolism, arginine and proline metabolism, histidine metabolism, insulin secretion, and lysine degradation, among other metabolic processes were associated with a significant portion of the candidate genes, according to GO and KEGG analyses (
Figure S6).
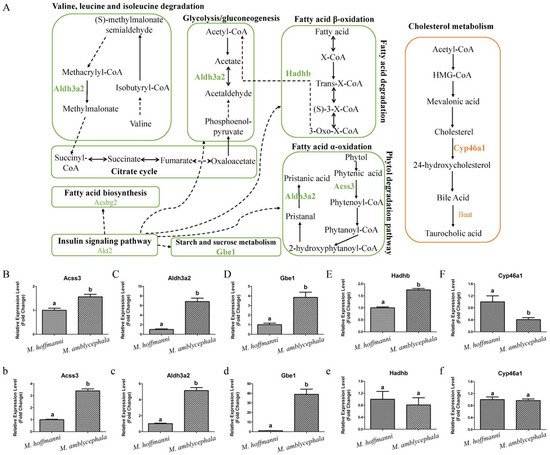
M. amblycephala mainly feeds on high-fiber and low-energy aquatic vascular plants. The candidate genes of
M. amblycephala were enriched in fatty acid degradation and corresponding upstream and downstream pathways. For instance,
Aldh3a2 and
Acss3 genes in fatty acid α-oxidation,
Hadhb gene in fatty acid β-oxidation,
Akt2 gene in insulin signaling pathway,
Aldh3a2 gene in glycolysis/gluconeogenesis,
Gbe1 gene in starch and sucrose metabolism,
Acsbg2 gene in fatty acid biosynthesis, and
Aldh3a2 gene in valine, leucine, and isoleucine degradation (
Figure 6A and
Table S7). Moreover, these genes exhibited a high expression pattern in the liver and/or spleen tissue of
M. amblycephala compared with
M. hoffmanni (
Figure 6B–E,b–e).
Figure 5. Genome-wide inference of selection sweeps on chromosomes during the diet adaptation of M. amblycephala (A) and M. hoffmanni (B). MA and MH refer to M. amblycephala and M. hoffmanni. Gbe1, Akt2, and Aldh3a2 were identified from the comparison between M. amblycephala and M. skolkovii, Acss3, and Acsbg2 from the comparison between M. amblycephala and M. pellegrini, Hadhb from the comparison between M. amblycephala and M. hoffmanni, and Cyp46a1, Tas1r1, and Baat from the comparison between M. hoffmanni and M. amblycephala. The black curve indicates the nucleotide polymorphism ratio (PiR) analysis, and red curve indicates extended haplotype homozygosity between populations (XP-EHH) analysis. The green and pink boxes represent the position of the selected genes on the chromosomes.
Figure 6. Metabolism pathways and expression pattern of candidate genes. (A) Candidate genes of M. amblycephala and M. hoffmanni enriched in the metabolism pathways. The lines and selected genes in M. amblycephala and M. hoffmanni are indicated in green and pink, respectively. The dashed lines used to connect KEGG pathways represent indirect relationships. (B–F) The expression pattern of candidate genes (Acss3, Aldh3a2, Gbe1, Hadhb, and Cyp46a1) in the liver tissue of M. amblycephala and M. hoffmanni. (b–f) The expression pattern of candidate genes (Acss3, Aldh3a2, Gbe1, Hadhb, and Cyp46a1) in the spleen tissue of M. amblycephala and M. hoffmanni. Different letters indicate a significant difference (p < 0.05).
Among the three omnivorous
Megalobrama species, the food composition of
M. hoffmanni is rich in zoobenthos. The common regions of top 5% Fst, High top 5% PiR, and Low top 5% XP-EHH were the selected regions inferred from a comparison of
M. hoffmanni and
M. amblycephala, which covered a total of 75 selected regions with a length of 4.14 Mb and including 126 genes in
M. hoffmanni. These genes were found to be abundant in cellular and metabolic processes including taste transduction, fatty acid elongation, biosynthesis of unsaturated fatty acids, and biosynthesis of amino acids according to GO and KEGG enrichment analysis (
Figure S7). Interestingly, we found some candidate genes (
Cyp46a1 and
Baat) involved in cholesterol metabolism (
Figure 5B,
Figure 6A,
Figure S5B and Table S7). The results indicated that compared with
M. amblycephala, the
Cyp46a1 gene was highly expressed in the liver of
M. hoffmanni, but not in the spleen (
Figure 6F,f). Furthermore, functional annotation indicated that the umami taste receptor gene
Tas1r1 was abundant in the
M. hoffmanni sensory system.
This entry is adapted from the peer-reviewed paper 10.3390/biology11020186