You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Green & Sustainable Science & Technology
At present, the majority of available road and jet biofuels are produced from oleochemical feedstocks that include vegetable oils and biowastes such as waste cooking oils and animal fats. Additionally, one of the most promising ways to achieve long-term environmental goals is to sustainably use lignocellulosic residues. These resources must be treated through a deoxygenation process and subsequent upgrading processes to obtain high-quality road and jet biofuels.
- biowaste
- deoxygenation
- nanomaterial
- biofuel
- oleochemical
1. Introduction
At present, different economic sectors produce considerable amounts of biowastes and lignocellulosic residues that are not fully exploited or properly disposed of, but it is estimated that, by 2050, renewable sources will be the main resources used in energy production, and among these, biomass will play a significant role in the production of zero emission fuels [1]. The biofuel sector is making a significant contribution in improving the capability to transform biowastes into high-quality road and jet biofuels, which is highly advantageous for the development of a more sustainable global economy. In fact, the majority of available road and jet biofuels are produced from oleochemical feedstocks that include vegetable oils and biowastes such as waste cooking oils (WCOs), animal fats, and tallow. However, these resources are characterized by having a high content of oxygen, unlike fossil fuels which are oxygen free. Consequently, biowastes and lignocellulosic residues must be treated through a deoxygenation process and subsequent upgrading processes to obtain high-quality road and jet biofuels [2][3].
2. Characteristics of Diesel and Jet Biofuels
Diesel fuel is a complex mixture produced by the distillation of crude oil. It consists of hydrocarbons with carbon numbers predominantly in the range of C9–C20 and boiling points in the range of ~163–357 °C [4]. Diesel fuel contains approximately 75% aliphatic hydrocarbons, mainly straight- and branched-chain paraffins (alkanes), and about 25% aromatic hydrocarbons (with one or more aromatic rings and alkyl side chains). The typical atomic mass concentrations are about 86% C, 14% H, and a minor fraction of sulfur depending on the crude oil source and cleaning quality [5]. Renewable diesel obtained by hydroprocessing of oleochemical feedstocks also contains straight- and branched-chain paraffins (alkanes), however, it is practically free of aromatics. Due to its paraffinic composition, renewable diesel can be blended with diesel fuel without limitations set by vehicle technology or fuel logistics. Moreover, the presence of isoparaffins obtained by an additional isomerization step provides renewable diesel with good cold properties. For example, during isomerization, the melting point of n-paraffins, i.e., around 28 °C for C18, can be adjusted down to −40 °C to meet the requirements of fuels used in severely cold climates [6]. In general, for improved low temperature properties, shorter chain, isomerized compounds are more desirable than long-chain alkanes, although an excessive presence of shorter chain, isomerized species provides a lower number of cetane. Hexadecane (cetane) is the high-quality reference compound on the cetane scale, and it is related to the ignition quality of diesel fuel. [7].
Conversely, conventional jet fuels are mainly composed of C8–C16 hydrocarbons, categorized into four principal hydrocarbon groups: n-paraffins (alkanes), iso-paraffins (branched iso-alkanes), cyclo-paraffins (naphthenes), and aromatics, with a content of around 20%, 40%, 20%, and 20%, respectively [8][9]. The content of each of these compounds has a direct effect on the properties of the fuel. For example, the presence of n-paraffins ensures the energy density, while iso-paraffins and cyclo-paraffins are responsible for decreasing the freezing point, since they have a significantly lower freezing point than that of n-paraffins with the same carbon number. Aromatics also help to reduce the freezing point and to enhance the shrinkage of aged elastomer seals, minimizing potential fuel leakage issues, however, excessive use of aromatics can reduce the net heat of combustion and can lead to more soot formation [10][11][12]. The ASTM D1655 standard classifies jet fuels according to their properties as jet fuels for commercial airplanes (Jet A and Jet A-1) and jet fuels for military aircrafts (JP-5 and JP-8). The only difference between Jet A and Jet A-1 is the freezing point, which is −40 °C and −47 °C, respectively. Jet A fuel is generally used in the United States, while Jet A-1 is adopted in the rest of the world [13]. Although some test flights have operated with 100% bio-synthetic paraffinic kerosene (Bio-SPK), ASTM D7566 has restricted its use in mixture with jet fuel of fossil origin to a maximum of 50% to guarantee effective operation of aircrafts. Additionally, the ASTM D7566 has stated that the final composition of Bio-SPK must be at least 99.5% in carbon and hydrogen. Consequently, the removal of oxygen is highly important to increase biofuel stability, miscibility with conventional fuels and the H/C molar ratio [14][15][16].
4. Deoxygenation of Oleochemical Feedstocks in a Free-Hydrogen Environment
At present, the most widely used route to produce road and jet biofuels from oleochemical feedstocks uses hydrogen in the process (hydrodeoxygenation). When hydrodeoxygenation is applied to free fatty acids (FFA), it allows the removal of two oxygen atoms involving two sequential C-O cleavage steps by sequential hydrogenations, giving rise to the formation of a linear hydrocarbon and two H2O molecules. As described earlier, oleochemical feedstocks and the use of hydrogen and catalysts in the hydrodeoxygenation process, can represent 50% and 40% of the total production cost, respectively (Table 4 shows the variable operating cost of a hydrotreating facility). Therefore, from an economic point of view, it is highly desirable to widen the range of feedstocks, use less costly catalysts, and avoid the use of hydrogen in the process. In this regard, the deoxygenation of FFA can occur directly without using hydrogen in the process through reactions such as decarboxylation, decarbonylation, and ketonization. Selective decarboxylation applied to FFA involves one C-C cleavage step, which results in the removal of oxygen atoms producing CO2 and a paraffin hydrocarbon (n-alkane with one less carbon atom than the starting fatty acid); while selective decarbonylation involving C-C and C-O cleavage steps leads to the formation of CO, H2O and an olefinic hydrocarbon (the corresponding alkene) [17][18]. On the other hand, the ketonization reaction converts two molecules of FFA into a ketone releasing CO2 and H2O. The ketonization reaction has proven to be highly efficient for the selective removal of oxygen from FFA because three of four oxygen atoms are removed eliminating only one carbon atom, and consequently, a lesser amount of hydrogen is required to remove the last oxygen atom of the ketone and produce linear alkanes [19][20]. Tests performed on CI engines have shown that deoxygenated vegetable oils can provide better engine performance and emissions as compared with diesel fuel. [21]. Figure 2 shows a representation of the four plausible reaction routes for the deoxygenation of stearic acid using hydrogen (hydrodeoxygenation) or in the absence of this gas (decarboxylation, decarbonylation, and ketonization).
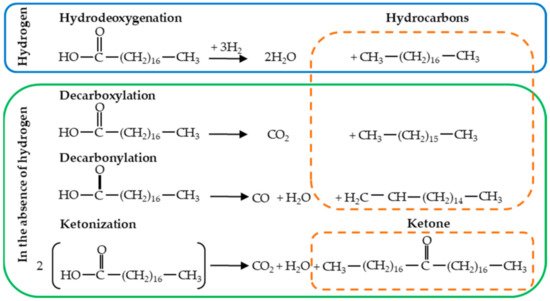
Figure 2. Representation of plausible reaction routes for the deoxygenation of stearic acid using hydrogen (hydrogenation) or in the absence of this gas (decarboxylation, decarbonylation, and ketonization).
Table 4. Variable operating cost of a hydrotreating facility with a capacity of 600 tons/day [22].
| Material | Consumption | Unit Cost (USD Per Unit) |
|---|---|---|
| Feedstock | 25,000.00 kg/hr | 0.80/kg |
| Natural gas | 35,527.00 MJ/h | 0.39/m3 |
| Cooling water | 345,700.00 kg/hr | 0.41/m3 |
| Electricity | 2496.76 kWh | 0.07/kWh |
| Hydrogen | 676.84 kg/hr | 3.51/kg |
| Hydroprocessing catalyst | 25,000.00 kg/year | 3266.10/kg |
| Hydrocracking catalyst | 5991.34 kg/year | 5935.39/kg |
| Wastewater treatment chemicals | 21.75 kg/hr | 6.81/kg |
| Propane | 1771.55 kg/hr (production) | 1.00/kg |
| Heavy oil | 809.10 kg/hr (production) | 0.60/kg |
The materials that have been used as catalysts in the deoxygenation of oleochemical feedstocks can be divided mainly into four groups: catalysts containing noble metals (e.g., Pd, Pt, and Ru); transition metals (e.g., Ni, Co, and Mo); alkaline earth metals (e.g., Mg and Ca); and zeolites (ZSM-5). Metal catalysts can be used in the form of pure metals (e.g., Mo, Ni, and Co); metallic mixtures (e.g., CoMo and NiMo); metal oxides; metal phosphides; metal nitrides; or metal carbides [23]. Additionally, metal catalysts have typically been used with suitable supports such as metal oxides (TiO2, Al2O3, SiO2, CeO2, and ZrO2); zeolites (ZSM-5, HY, and H-Beta); mesoporous materials (MCM-41, SAPO-11, SBA-15, Al-SBA-15, and Al-MCM-41); and activated carbon (AC). Among these, Al2O3 is one of the most common supports, which in combination with metals, has shown excellent deoxygenation properties due to its acidic property; however, the high acidity of Al2O3 also causes a high tendency for coke deposition on the catalyst surface that eventually leads to instability and deactivation [24].
Transition metals, such as Pt and Pd, have proven to be excellent catalysts for the deoxygenation process; however, their high cost represents an important drawback from an economic point of view [25]. Consequently, less expensive materials such as Ni, Mg, and Ca have been proposed. In fact, it has been reported that feedstocks such as non-edible oil (Jatropha curcas oil) and waste cooking oil (WCO) can be successfully deoxygenated and converted into hydrocarbons in a hydrogen-free atmosphere by promoting decarboxylation and decarbonylation reactions using γ-Al2O3, CaO, and Mg-Al mixed oxides (calcined hydrotalcite) as catalysts. In experiments that were mainly carried out in a batch setup (up to 110 bar), the possibility of obtaining a liquid fuel potentially suitable for use in the transportation sector has been verified, with yields over 80 wt.% and a high proportion of hydrocarbons (i.e., around 83%) of mainly C8–C18; additionally, significant improvements in terms of heating value (44 MJ/kg), viscosity (4 cSt), and sulfur content (below 10 ppmw) were achieved [26][27][28]. In addition, the performance of Mg-Al mixed oxides has also been compared with other activated hydrotalcite-derived catalysts (FeAl, ZnAl, and NiAl) in the deoxygenation of oleic acid under atmospheric pressure. Mg-Al and Ni-Al mixed oxide catalysts both produced a high yield of hydrocarbons, i.e., 81% and 89%, respectively. Although it was evidenced that Mg-Al mixed oxides provided greater selectivity towards C8–C12 hydrocarbons (30%) as compared with Ni-Al mixed oxides (17%), for which the selectivity towards hydrocarbons in the range of C13–C20 was predominant (30%). These results highlighted that hydrocarbon yield and selectivity of the deoxygenation products were predominantly influenced by the acid-base properties of the catalyst [29].
Although Ni-based catalysts have shown good potential for the deoxygenation process, they have been identified to be prone to undergo coking mainly due to their acidity properties, and subsequently, high activity in cracking reactions [30]. Consequently, to neutralize the acidity properties of Ni-based catalysts, their integration with basic metal materials such as Co and Ca oxides has been proposed. For example, the performance of NiO-CaO/SiO2-Al2O3 (5 wt.% CaO metal content) has been evaluated during the deoxygenation of triolein, WCO, Jatropha curcas oil, and palm fatty acid distillate (PFAD). However, although all feedstocks were effectively deoxygenated, producing a high percentage of hydrocarbons (>74%) with a selectivity mainly towards C15–C17 hydrocarbons (>54%), the results also showed that using NiO-CaO/SiO2-Al2O3 as a catalyst, in all cases, resulted in the formation of a significant amount of coke (around 14 wt.%) [31]. In addition, the performance of Co-Ca/SiO2-Al2O3 (10 wt.% of Co) has been evaluated during the deoxygenation of triolein, Ceiba oil, and Sterculia oil. In general, it was possible to obtain a high hydrocarbon yield comprised between 73% and 86% and, when triolein was used as a raw material, the selectivity of the C15–C17 hydrocarbons reached 48%, however, high production of char was also evidenced (31 wt.%). Furthermore, the results showed the presence of a significant carbon amount (33–63 wt.%) on the reactivated Co-Ca/SiO2-Al2O3 catalyst surfaces implying a slight gradual reduction in deoxygenation activity and coke formation [32]. In addition, a study was conducted on the catalytic performance of mono-functional metals supported on activated carbon derived from coconut shell (Co/AC and Mn/AC) as catalysts in the deoxygenation of PFAD. The results showed that the Co/AC catalyst exhibited high deoxygenation activity, with a hydrocarbon yield of 90% and C15–C17 hydrocarbon selectivity of 72%, however, the mass fractions of the liquid products were relatively low, i.e., between 40 wt.% and 52 wt.%. The low yield of liquid products was mainly attributed to the mild cracking of the deoxygenated products, leading to the formation of significant amounts of volatile materials (38 wt.%), as well as the formation of char (19 wt.%), while the high hydrocarbon yield was attributed to the synergistic effect between the CoO and the active carbon support, which promoted the characteristics of the acid–base sites [33]. Additionally, a study reported the development of mixed metal oxide Ni-Ag supported on activated carbon (AC) derived from coconut fiber residues (CFR) for producing diesel-like hydrocarbons via the deoxygenation of Jatropha Curcas oil. Under optimal process conditions it was possible to obtain a hydrocarbon yield of up to 95% with 83% selectivity towards C15 and C17 hydrocarbons, produced predominantly by decarbonylation rather than decarboxylation. Despite the high hydrocarbon yield, the experimental results showed that the mass fraction of liquid product was not greater than 42 wt.%, with the drawback of the formation of char and residue (29 wt.%), which blocked the accessibility to the active sites and diminished the efficiency of the catalyst. Ni-Ag/AC was reused for five consecutive runs without a drastic reduction in hydrocarbon yield, but with inevitable coke formation in the spent catalyst (2.5 wt.%). Interestingly, the study also highlighted that an Ni-rich catalyst facilitated the conversion of triglycerides to fatty acids rather than the conversion of fatty acid to hydrocarbon fractions, suggesting that an Ni-rich catalyst favors cracking and polymerization reactions. Furthermore, it was evidenced that there was no correlation between the catalyst’s textural properties and the increment of hydrocarbons, instead, the deoxygenation activity strongly depended on the chemical nature of the catalyst, specifically, in relation to the distribution of strong and weak acidic sites [34].
5. Deoxygenation of Oleochemical Feedstocks Using Nanomaterials as Catalysts
As a strategy to optimize the deoxygenation of oleochemical feedstocks, it has been proposed to test the use of nanocatalysts such as transition metal oxides supported on TiO2 (WO/Pt/TiO2), which have been tested during the deoxygenation of stearic acid and FFA from Jatropha curcas oil. Tungsten addition to Pt nanoparticles supported on TiO2 showed remarkably enhanced performance of the catalyst, providing a conversion of 86% and more than 90% for the deoxygenation of stearic acid and FFA, respectively, which was more than two times higher than the conversion degree achieved using a Pt/TiO2 catalyst. The liquid fuel obtained was composed of saturated hydrocarbons, mainly C17 and C15, meaning that decarboxylation and decarbonylation were the dominant reaction routes. In the study, the effect of environment gases (N2, 10% H2/N2, and H2) on the degree of deoxygenation of FFA was also measured. When N2 was used, the degree of deoxygenation was decreased to 55% after 9 h (time on stream), while using hydrogen-contained gases, preferably 100% hydrogen gas, the degree of the deoxygenation was above 90% for more than 20 h (time on stream), demonstrating that the hydrogen supply on the catalyst was favorable for preserving catalytic performance [35]. Moreover, it is noteworthy that carbon could help to reduce the production cost of the deoxygenation process because it is a widely available material typically used as a support for transition metals [36]. It has been proposed to study the deoxygenation of heptanoic acid over Pt nanoparticles supported on three different carbon supports (Pt/Norit activated carbon, Pt/Silicon carbon, and Pt/Vulcan carbon) prepared by an incipient wetness impregnation (IWI) method. It was shown that this method was unsuccessful for obtaining a high dispersion of Pt on Silicon carbon and Vulcan carbon, however, it was possible to obtain a high Pt dispersion on Norit carbon (31%). Although it was possible to obtain high selectivity towards the formation of olefin hydrocarbons by using Pt/Norit C as the catalyst, through decarbonylation as the primary reaction, the heptanoic acid conversion was low [37]. In a subsequent study, the use of an alcohol reduction method was tested to deposit Pd nanoparticles on Vulcan carbon and Silicon carbon, however, it was verified that the impregnation method did not influence the catalytic performance or stability of the resulting Pd nanoparticles [38].
It has been identified that an alkaline earth metal-based nanocatalyst such as (Ca(OH)2) can provide better reactivity and product selectivity as compared with a CaO catalyst, mainly due to its superior textural properties (surface area, pore volume, and pore diameter) and basicity. Therefore, the deoxygenation of triolein using a Ca(OH)2 nanocatalyst derived from low-cost natural waste shells has been studied. As a result, it was identified that the shape of waste shell-derived CaO, which initially appeared in irregular aggregate forms, changed to cubic-like nanostructures after being treated with surfactants (ethylene glycol (EG) and N-cetyl-N,N,N-trimethylammonium bromide (CTAB) solutions) and wet sonochemical effect. In addition, it was possible to verify that using Ca(OH)2-EG and Ca(OH)2-CTAB nanocatalysts, the deoxygenation process was carried out successfully and the initial O/C ratio of triolein was strongly reduced from 0.31 to around 0.07–0.08, while the H/C ratio was maintained from 0.180 to around 0.175–0.177.
Overall, bimetallic acid-based nanocatalysts have been shown to play important roles in the deoxygenation of lipid-rich sources by promoting the development of reactions such as decarboxylation and decarbonylation under a hydrogen-free atmosphere and providing, in many cases, a higher conversion and selectivity of hydrocarbons as compared with mesoporous catalysts. This is due to the superior textural properties (e.g., surface area, pore volume, and pore diameter) of the nanocatalysts as well as due to the coexistence of basic sites and weak/medium acid sites in the catalytic systems. In fact, the type of metal, ratio composition, size, and shape are all essential parameters to achieve desired reactions and products. Additionally, the lower tendency of carbon-based nanocatalysts to form coke allows them to have better catalyst stability, avoiding major negative effects such as the occurrence of side reactions on the deoxygenation process. In addition, the uniform shape of these supports facilitates the diffusion of biomass-derived compounds as compared with conventional catalysts. As highlighted in the next section, nanocatalysts can be used to valorize whole lignocellulosic materials (lignin, cellulose, and hemicellulose), and to more efficiently promote the extraction of their main compounds, as well as the synthesis of long-chain oxygenates via C–C coupling reactions and their subsequent deoxygenation in the absence of hydrogen.
This entry is adapted from the peer-reviewed paper 10.3390/pr10020187
References
- Energy Technology Perspectives 2017, Executive Summary. Available online: https://www.iea.org/reports/energy-technology-perspectives-2017 (accessed on 3 February 2019).
- Olusola, J.O.; Adediran, M.M.; Oluseyi, A.K.; Ajao, U.L. Processing of triglycerides to diesel range hydrocarbon fuels: Easily practicable small-scale approach. Energy Environ. 2009, 20, 1325–1341.
- Srivastava, A.; Prasad, R. Triglycerides-based diesel fuels. Renew. Sustain. Energy Rev. 2000, 4, 111–113.
- Gad, S.C. Diesel Fuel. In Encyclopedia of Toxicology, 2nd ed.; Wexler, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 19–22.
- Huth, M.; Heilos, A. Fuel flexibility in gas turbine systems: Impact on burner design and performance. In Modern Gas Turbine Systems; Jansohn, P., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 635–684.
- Neste Renewable Diesel Handbook. Available online: https://www.neste.com/sites/neste.com/files/attachments/neste_renewable_diesel_handbook.pdf (accessed on 5 February 2019).
- Knothe, G. Biodiesel and renewable diesel: A comparison. Prog. Energy Combust. Sci. 2010, 36, 364–373.
- Wei, H.; Liu, W.; Chen, X.; Yang, Q.; Li, J.; Chen, H. Renewable bio-jet fuel production for aviation: A review. Fuel 2019, 254, 115599.
- Gutiérrez-Antonio, C.; Gómez-Castro, F.I.; De Lira-Floresa, J.A.; Hernández, S. A review on the production processes of renewable jet fuel. Renew. Sustain. Energy Rev. 2017, 79, 709–729.
- Duong, L.H.; Fujita, O.; Reksowardoj, I.K.; Soerawidjaja, T.H.; Neonufa, G.F. Experimental investigation of the effects of cycloparaffins and aromatics on the sooting tendency and the freezing point of soap-derived biokerosene and normal paraffins. Fuel 2016, 185, 855–862.
- Blakey, S.; Rye, L.; Wilson, C.W. Aviation gas turbine alternative fuels: A review. Proc. Combust. Inst. 2011, 33, 2863–2885.
- Al-Nuaimi, I.A.; Bohra, M.; Selam, M.; Choudhury, H.A.; El-Halwagi, M.M.; Elbashir, N.O. Optimization of the Aromatic/Paraffinic Composition of Synthetic Jet Fuels. Chem. Eng. Technol. 2016, 39, 2217–2228.
- Khan, S.; Lup, A.N.K.; Qureshi, K.M.; Abnisa, F.; Daud, W.M.A.W.; Patah, M.F.A. A review on deoxygenation of triglycerides for jet fuel range hydrocarbons. J. Anal. Appl. Pyrolysis 2019, 140, 1–24.
- Why, E.S.K.; Ong, H.C.; Lee, H.V.; Gan, Y.Y.; Chen, W.H.; Chong, C.T. Renewable aviation fuel by advanced hydroprocessing of biomass: Challenges and perspective. Energy Convers. Manag. 2019, 199, 112015.
- Goh, B.H.H.; Chong, C.T.; Ge, Y.; Ong, H.C.; Ng, J.H.; Tian, B.; Ashokkumar, V.; Lim, S.; Seljak, T.; J’ozsa, V. Progress in utilisation of waste cooking oil for sustainable biodiesel and biojet fuel production. Energy Convers. Manag. 2020, 223, 113296.
- Lokesh, K.; Sethi, V.; Nikolaidis, T.; Goodger, E.; Nalianda, D. Life cycle greenhouse gas analysis of biojet fuels with a technical investigation into their impact on jet engine performance. Biomass Bioenergy 2015, 77, 26–44.
- Kim, S.K.; Han, J.H.; Lee, H.; Yum, T.; Kim, Y.; Kim, J. Production of renewable diesel via catalytic deoxygenation of natural triglycerides: Comprehensive understanding of reaction intermediates and hydrocarbons. Appl. Energy 2014, 116, 199–205.
- Gosselink, R.W.; Hollak, S.A.W.; Chang, S.; Haveren, J.; Jong, K.P.; Bitter, J.H.; Es, D.S. Reaction Pathways for the Deoxygenation of Vegetable Oils and Related Model Compounds. ChemSusChem 2013, 6, 1576–1594.
- Oliver-Tomas, B.; Renz, M.; Corma, A. Ketone Formation from Carboxylic Acids by Ketonic Decarboxylation: The Exceptional Case of the Tertiary Carboxylic Acids. Chem. Eur. J. 2017, 23, 12900–12908.
- Romero, M.; Pizzi, A.; Toscano, G.; Casazza, A.; Busca, G.; Bosio, B.; Arato, E. Deoxygenation of Non-Edible Vegetable Oil to Produce Hydrocarbons Over Mg-Al Mixed Oxides. Chem. Eng. Trans. 2018, 64, 121–126.
- Pattanaik, B.P.; Misra, R.D. Experimental studies on production of deoxygenated vegetable oils and their performance evaluation in a compression ignition engine. Biomass Convers. Biorefin. 2018, 8, 899–908.
- Hsu, K.; Wang, W.; Liu, Y. Experimental studies and techno-economic analysis of hydro-processed renewable diesel production in Taiwan. Energy 2018, 164, 99–111.
- Lup, A.N.K.; Abnisa, F.; Daud, W.M.A.W.; Aroua, M.K. A review on reactivity and stability of heterogeneous metal catalysts for deoxygenation of bio-oil model compounds. J. Ind. Eng. Chem. 2017, 56, 1–34.
- Ooi, X.Y.; Gao, W.; Ong, H.C.; Lee, H.V.; Juan, J.C.; Chen, W.H.; Lee, K.T. Overview on catalytic deoxygenation for biofuel synthesis using metal oxide supported catalysts. Renew. Sustain. Energy Rev. 2019, 112, 834–852.
- Santillan-Jimenez, E.; Crocker, M. Catalytic deoxygenation of fatty acids and their derivatives to hydrocarbon fuels via decarboxylation/decarbonylation. J. Chem. Technol. Biotechnol. 2012, 87, 1041–1050.
- Romero, M.; Pizzi, A.; Toscano, G.; Bosio, B.; Arato, E.; Study of an innovative process for the production of biofuels using non-edible vegetable oils. Chem. Eng. Trans. 2014, 37, 883-888, https://doi.org/10.3303/CET1437148.
- Romero, M.; Pizzi, A.; Toscano, G.; Casazza, A.; Busca, G.; Bosio, B.; Arato, E.; Preliminary experimental study on biofuel production by deoxygenation of Jatropha oil. Fuel Process. Technol. 2015, 137, 31–37 2015, 137, 31-37, https://doi.org/10.1016/j.fuproc.2015.04.002.
- Romero, M.; Pizzi, A.; Toscano, G.; Busca, G.; Bosio, B.; Arato, E.; Deoxygenation of waste cooking oil and non-edible oil for the production of liquid hydrocarbon biofuels. J. Waste Manag. 2016, 47, 62-68, https://doi.org/10.1016/j.wasman.2015.03.033.
- Abidin, S.N.Z.; Lee, H.V.; Asikin-Mijan, N.; Juan, J.C.; Rahman, N.A.; Mastuli, M.S.; Taufiq-Yap, Y.H.; Kong, P.S. Ni, Zn and Fe hydrotalcite-like catalysts for catalytic biomass compound into green biofuel. Pure Appl. Chem. 2019, 92, 587–600.
- Morgan, T.; Santillan-Jimenez, E.; Harman-Ware, A.E.; Ji, Y.; Grubb, D.; Crocker, M. Catalytic deoxygenation of triglycerides to hydrocarbons over supported nickel catalysts. Chem. Eng. J. 2012, 189, 346–355.
- Asikin-Mijana, N.; Lee, H.V.; Marliza, T.S.; Taufiq-Yap, Y.H. Pyrolytic-deoxygenation of triglycerides model compound and non-edible oil to hydrocarbons over SiO2-Al2O3 supported NiO-CaO catalysts. J. Anal. Appl. Pyrol. 2018, 129, 221–230.
- Asikin-Mijana, N.; Lee, H.V.; Juana, J.C.; Noorsaadah, A.R.; Ong, H.C.; Razali, S.M.; Taufiq-Yap, Y.H. Promoting deoxygenation of triglycerides via Co-Ca loaded SiO2-Al2O3 Catalyst. Appl. Catal. A-Gen. 2018, 552, 38–48.
- Gamala, M.S.; Asikin-Mijana, N.; Arumugama, M.; Rashidc, U.; Taufiq-Yap, Y.H. Solvent-free catalytic deoxygenation of palm fatty acid distillate over cobalt and manganese supported on activated carbon originating from waste coconut shell. J. Anal. Appl. Pyrol. 2019, 144, 104690.
- Asikin-Mijan, N.; Ooi, J.M.; AbdulKareem-Alsultan, G.; Lee, H.V.; Mastuli, M.S.; Mansir, N.; Alharthi, F.A.; Alghamdi, A.A.; Taufiq-Yap, Y.H. Free-H2 deoxygenation of Jatropha curcas oil into cleaner diesel-grade biofuel over coconut residue-derived activated carbon catalyst. J. Clean. Prod. 2020, 249, 119381.
- Choi, I.; Lee, J.; Kim, C.; Kim, T.; Lee, K.; Hwang, K. Production of bio-jet fuel range alkanes from catalytic deoxygenation of Jatropha fatty acids on a WOx/Pt/TiO2 catalyst. Fuel 2018, 215, 675–685.
- Abdulkareem-Alsultan, G.; Asikin-Mijan, N.; Lee, H.V.; Rashid, U.; Islam, A.; Taufiq-Yap, Y.H. A review on thermal conversion of plant oil (edible and inedible) into green fuel using carbon-based nanocatalyst. Catalysts 2019, 9, 350.
- Lopez-Ruiz, J.A.; Davis, R.J. Decarbonylation of heptanoic acid over carbon-supported platinum nanoparticles. Curr. Green Chem. 2014, 16, 683–694.
- Lopez-Ruiz, J.A.; Pham, H.N.; Datye, A.K.; Davis, R.J. Reactivity and stability of supported Pd nanoparticles during the liquid-phase and gas-phase decarbonylation of heptanoic acid. Appl. Catal. A-Gen. 2015, 504, 295–307.
This entry is offline, you can click here to edit this entry!
