Graphene is an allotrope of carbon consisting of a single layer of atoms arranged in a two-dimensional honeycomb lattice. Carbon atoms are bonded with a covalent sp2bond with a single free electron, which accounts for the conductivity of graphene. Graphene is attracting great interests from the physical, chemical, and biomedical fields as a novel nanomaterial with exceptional physical properties, including extremely high thermal conductivity, excellent electrical conductivity, high surface-to-volume ratio, remarkable mechanical strength, and biocompatibility.
- graphene
- graphene nanocomposites
- graphene characterizations
- polymer nanocomposites
- mechanical properties of graphene nanocomposites
1. Introduction
This paper provides a critical review of the synthesis, properties and characterizations perspectives of recent advances in graphene-based nanocomposites. Section 2 presents an overview of the importance of the graphene properties and prospect applications in smart phones, ultra-thin flexible displays, hydrogen storage, transparent touchscreens, chemical sensors, biosensors, and super-fast transistors. Section 3 and Section 4 summarize the most frequent graphene synthesis techniques including mechanical exfoliation, liquid-phase exfoliation and chemical synthesis technique. They also highlight the polymer nanocomposite processing methods and the morphological states for graphene-based polymer nanocomposites.
A critical review of the characterization of graphene and graphene nanocomposites was presented in Section 5 . Detailed research results of graphene characterization from recent literature are thoroughly discussed. Different types of microscopic and spectroscopic characterization methods to obtain structural and morphological data are presented. Mechanical properties of graphene-based nanocomposites are thoroughly discussed in Section 6 . In addition, a summary from recent research that exemplifies the effect of graphene’s filler on the improvement of mechanical properties of graphene-based polymer nanocomposites is also thoroughly discussed. Two major tables summarizing the reinforcing effect of graphene-based materials on mechanical properties and thermal conductivity have been constructed. The thermal properties of graphene and graphite nanocomposites are subsequently discussed in Section 7 . The variation in thermal conductivity with different forms of graphene and graphite nanocomposites from recent research are summarized.
2. Graphene
Carbon has several allotropes, which can be classified according to the type of chemical bond related with hybridization (sp, sp 2, sp 3): zero-dimensional sp 2 fullerenes, the two-dimensional sp 2 honeycomb lattice of graphene, or three-dimensional sp 3 crystals—diamond [1][2][3][4]. Each allotrope has different electronic and mechanical properties. Graphene, fullerenes, and carbon nanotubes (CNTs) are emerging new materials with superior properties ( Figure 1 ). The great versatility of carbon materials arises from the strong dependence of their physical properties on the ratio of sp 2 ~graphitelike to sp 3 ~diamondlike bonds [4]. There are many forms of sp 2-bonded carbons with various degrees of graphitic ordering, ranging from microcrystalline graphite to glassy carbon. Accordingly, these materials have been greatly investigated because of the exceptional mechanical and electronic properties.
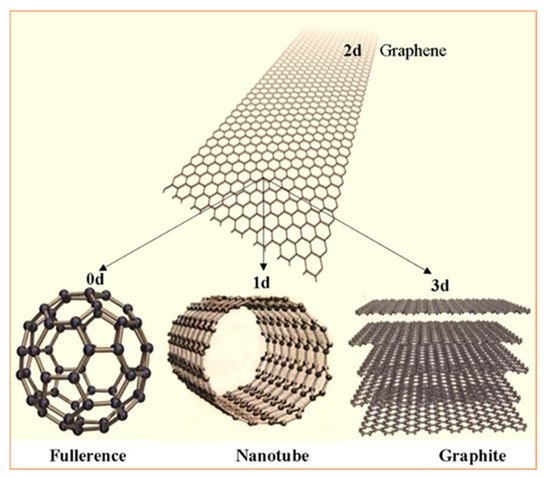
Graphene is an allotrope of carbon consisting of a single layer of atoms arranged in a two-dimensional honeycomb lattice [1]. Carbon atoms are bonded with a covalent sp 2 bond with a single free electron, which accounts for the conductivity of graphene. Graphene is attracting great interests from the physical, chemical, and biomedical fields as a novel nanomaterial with exceptional physical properties, including extremely high thermal conductivity, excellent electrical conductivity [1][2][3][4][5], high surface-to-volume ratio, remarkable mechanical strength, and biocompatibility [6][7][8][9][10][11]. Graphene possesses unique electronic properties and is recognized as the most thermally conductive known material [12][13][14][15][16][17]. Experimental results show that graphene has a remarkably high electron mobility at room temperature [12][18], and has been considered as an alternative in transistor circuitry. The electron mobility in graphene is almost 200 times higher than Si and 4 times larger than III–V semiconductors [15]. This would make graphene a very attractive material for high-speed transistors.
Since its discovery in 2004, graphene has become the center of many research activities [1][9][19][20][21][22][23][24][25][26][27][28][29][30]. It is a unique type of carbon where every atom is accessible for chemical reaction because of its 2D structure. With a Young’s modulus (stiffness) of 1 TPa, it is the strongest material ever tested [8]. Graphene possesses other remarkable characteristics: electron mobility is 100× faster than silicon; its electrical conductivity is 13× better than copper; it conducts heat 2× better than diamond; and it has a high surface area of about 2630 m 2/gram. Over the past decade research on graphene increased dramatically because of new methods to produce and study it. Graphene and functionalized graphene (FG) have been successfully used in many applications including in smart phones, ultra-thin flexible displays [31], hydrogen storage [32], transparent touch-screens [33], chemical sensors effective at detecting explosives [34][35], biosensors, super-fast transistors [36][37][38], and so on. Graphene has been investigated for tissue engineering [39]. It has also been utilized as a reinforcing agent to enhance the mechanical properties of biodegradable polymeric nanocomposites for bone tissue applications.
Graphene reveals remarkable optical properties, which makes it very promising for photonic and optoelectronic applications [31][40][41]. It is nearly transparent to visible light as well as to UV and IR. Graphene can be used to conduct electricity away from the solar panel as part of a light and flexible solar panel. However, the proportion of the defects in the structure of graphene has a great influence on the physical and mechanical properties. Graphene nanocomposites (GNP) possess a high aspect ratio, which makes them ideal for reinforcement [42][43][44][45][46][47][48][49]. The set of remarkable properties of graphene-based systems has expanded into new fields of investigation. Graphene is truly a multi-disciplinary material, being researched in many different fields for various potential applications. The optical of graphene represents potential fields of significant research and application.
3. Graphene-Based Nanocomposites
Research on polymer nanocomposites (PNC) has been growing over the past decade due to their remarkable material properties, yield strength, toughness, electrical conductivity, thermal conductivity, and optical properties, and their applications are growing substantially [2][50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69]. Traditional composite structures contain a significant amount (~50 vol%) of filler bound in a polymer matrix—PNC typically—containing a small amount of inorganic particles (usually 1 to 3 wt%) and size less than 100 nm, with a very large surface area dispersed in the polymer matrix [68]. However, it has been shown that a graphene of micron-size could be made scalable to mass production [70]. This makes graphene-based composite materials appealing to a great number of applications [70].
Graphene possesses many desirable properties such as high strength and elastic modulus, high electrical and thermal conductivity, high aspect ratio, high thermal stability, high gas impermeability, and good dimensional stability [6][7][8][9][10][11][12]. Polymer properties can be dramatically improved by the addition of graphene at a low volume fraction. Moreover, graphene has a higher surface-to-volume ratio than CNT and can be used at a lower volume fraction than CNT. It is potentially more promising for improving many properties of polymer matrices.
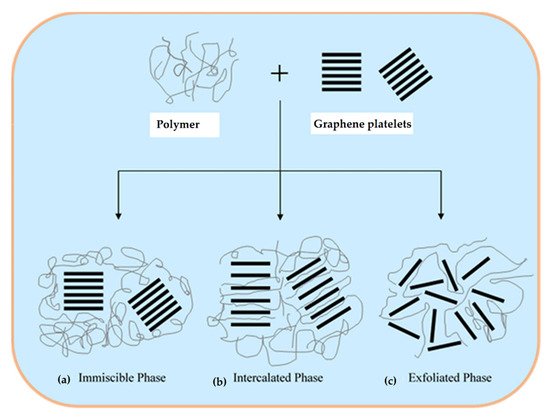
Depending upon the degree of dispersion of the nano-sized layer structure, polymer composites can be divided into three main categories: microcomposites, intercalated nanocomposites, and exfoliated nanocomposites [71][72][73]. In the microcomposites’ structure ( Figure 3 a), graphene sheets are dispersed inside the polymer matrix in the form of particles and the graphene platelets remain intact. When individual polymer chains are introduced between graphene layers, intercalated constructions are obtained ( Figure 3 b). In the exfoliated hybrids ( Figure 3 c), graphene layers are homogenously dispersed in the polymer matrix. The exfoliation configuration is the preferred morphology for nanocomposites as it maximizes the area of contact between the polymer and the filler and results in stronger bonding, and remarkable mechanical properties [74].
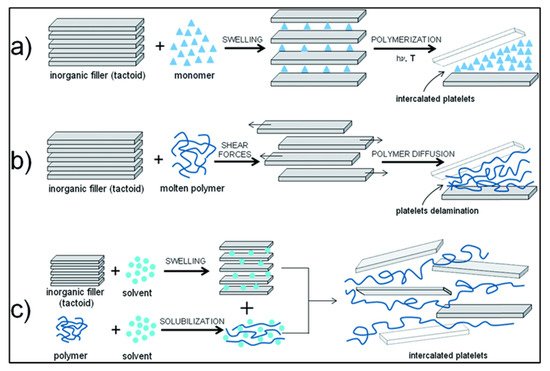
A more effective way to achieve interfacial adhesion between the filler and matrix is exfoliation adsorption ( Figure 4 c) [73][75][76][77]. Exfoliation adsorption, also called polymer intercalation from solution, or solution mixing, requires solvent compatibility between the filler and matrix [72]. Both the filler and matrix are dissolved then mixed together. The solvent causes the filler to swell, increasing interlayer space and allowing the polymer chains to intercalate in between the layers of the filler. The mixture is stirred and sonicated to obtain an even distribution and the solvent is removed by evaporation or precipitation. After solvent removal, polymer chains become entrapped between layers of the filler, forming a multilayer structure. This process is used for creating nanocomposites from polymers with low polarity but is not ideal for industrial use due to the large quantities of solvent required [78]. The solution mixing can be used to obtain polymer nanocomposites with a range of polymers, such as poly(methylmethacrylate) (PMMA) [79], polyurethane (PU) [80], and poly(vinyl alcohol) (PVA) [49].
4. Characterization of Graphene and Graphene Nanoplatelets (XGnPs)
Graphene is a material with outstanding properties, such as high specific surface area 2600 m 2/g, high mobility (15000 cm 2/V·s) [10][15], superior thermal conductivity (3000 W/m·K) [81], and extremely low permeability. Topological defects exist in large-area polycrystalline graphene [81][82][83][84], and are thought to play crucial roles in tailoring mechanical and physical properties of graphene [81][85][86][87][88]. Thus, characterization of graphene is an important step for understanding graphene’s properties. The overall electronic properties and the purity of a graphene sample are determined by the number of layers present. Characterizations of graphene encompass different types of microscopic and spectroscopic methods to obtain structural and morphological data of the synthesized graphene. Similarly, the characterization process is also related to determining of the purity and defects of graphene. Synthesis processes and/or processing parameters have a great effect on graphene’s purity. HRTEM and AFM are commonly used to determine number of layers of graphene. On the other hand, Raman spectroscopy is commonly employed to characterize the purity of graphene and to measure the number of its layers by detecting various crystal structures and bonding information. Furthermore, XPS and Raman spectroscopy are the fundamental methods for the measurement of graphene’s chemical purity and detection of functional groups attached to the graphene.
Figure 10 shows high-quality SEM images of graphene nanosheets produced for future application as a cathode material for sodium-ion batteries [89]. In this study, graphene oxide was synthesized by a modified Hummers’ method and reduced using a solid-state microwave irradiation method. The SEM images revealed a wrinkled stack of ultra-thin graphene oxide nanosheet with a porous morphology. A high-magnification SEM image ( Figure 10 b) shows a large number of nanopores between the nanosheets that are formed by gas evolution. Nanoporous carbon materials have attracted considerable technological interest due to their numerous applications, including improving the tensile strength of composites, as catalyst and sensor supports, as hydrogen-storage materials, and in electronic and electrochemical devices [1].
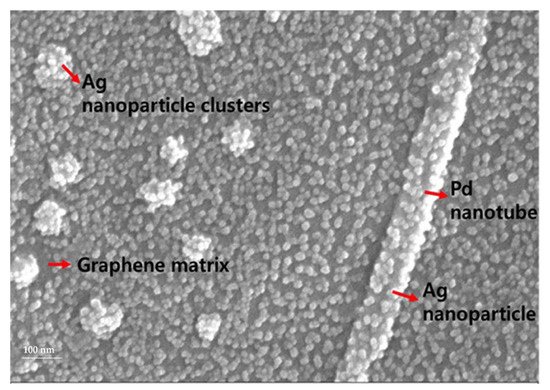
Graphene is quite a robust material in hydrogen sensors due to a possible improvement of its surface area and susceptibility of its electronic properties to the changes caused by adsorbing atoms and molecules including hydrogen. However, the pristine graphene sensitiveness to hydrogen is limited [34]. Sharma et al., developed a dual FET hydrogen gas sensor using graphene decorated Pd-Ag alloy nanoparticles for H 2 detection [90]. Figure 12 shows the SEM image of the graphene–Ag–Pd nanocomposites on the sensing area of the sensor platform. The integration between graphene, Pd and Ag can be visualized from the SEM image. Ag nanoparticles with the size of Ca. 17 nm and Pd nanoparticles at the size of Ca. 100 nm are uniformly and compactly embedded on the graphene layer. The morphology and nature of the Pd–Ag films grown on the graphene substrate are clearly shown in Figure 12 .
Due to strong interactions and van der Waals forces, exfoliated graphene sheets have a strong tendency to irreversibly aggregate or even restack, reverting to multilayer structures such as graphite [91]. Therefore, functionalization must be performed to reduce hydrophobicity, and to increase dispersion in organic and aqueous solutions. Covalent functionalization is the addition of molecules to graphene that results in the rehybridization of the sp 2 carbon atoms of the π network into a sp 3 configuration, resulting in adjustments of the innate physical and chemical properties of graphene [91].
This entry is adapted from the peer-reviewed paper 10.3390/polym13172869
References
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater 2007, 6, 183–191.
- Potts, J.R.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S. Graphene-based polymer nanocomposites. Polymer 2011, 52, 5–25.
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271.
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286.
- Young, R.; Kinloch, I.A.; Gong, L.; Novoselov, K. The mechanics of graphene nanocomposites: A review. Compos. Sci. Technol. 2012, 72, 1459–1476.
- Chandrasekaran, S.; Sato, N.; Tölle, F.; Mülhaupt, R.; Fiedler, B.; Schulte, K. Fracture toughness and failure mechanism of graphene based epoxy composites. Compos. Sci. Technol. 2014, 97, 90–99.
- Compton, O.C.; Nguyen, S. Graphene Oxide, Highly Reduced Graphene Oxide, and Graphene: Versatile Building Blocks for Carbon-Based Materials. Small 2010, 6, 711–723.
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385–388.
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669.
- Yasmin, A.; Daniel, I.M. Mechanical and thermal properties of graphite platelet/epoxy composites. Polymer 2004, 45, 8211–8219.
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924.
- Pallecchi, E.; Lafont, F.; Cavaliere, V.; Schopfer, F.; Mailly, D.; Poirier, W.; Ouerghi, A. High Electron Mobility in Epitaxial Graphene on 4H-SiC (0001) via post-growth annealing under hydrogen. Sci. Rep. 2014, 4, 4558.
- Chen, J.-H.; Jang, C.; Xiao, S.; Ishigami, M.; Fuhrer, M.S. Intrinsic and extrinsic performance limits of graphene devices on SiO2. Nat. Nanotechnol. 2008, 3, 206–209.
- Geim, A.K.; GHuang, X.; Lim, T.-T. Performance and Mechanism of a Hydrophobic−Oleophilic Kapok Filter for Oil/Water Separation. Desalination 2006, 190, 295–307.
- Kasap, S. Springer Handbook of Electronic and Photonic Materials; Springer Science & Busines Media: Berlin/Heidelberg, Germany, 2006.
- Novoselov, K.; Geim, A.K.; Morozov, S.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200.
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of indi-vidual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655.
- Bolotin, K. Electronic transport in graphene: Towards high mobility. Graphene 2014, 199–227.
- Nagashio, K.; Nishimura, T.; Kita, K.; Toriumi, A. Metal/graphene contact as a performance Killer of ultra-high mobility graphene analysis of intrinsic mobility and contact resistance. In Proceedings of the 2009 IEEE International Electron Devices Meeting (IEDM), Baltimore, MD, USA, 7–9 December 2009; pp. 1–4.
- Mattevi, C.; Colléaux, F.; Kim, H.; Lin, Y.-H.; Park, K.T.; Chhowalla, M.; Anthopoulos, T.D. Solution-processable organic dielectrics for graphene electronics. Nanotechnology 2012, 23, 344017.
- Aristov, V.Y.; Urbanik, G.; Kummer, K.; Vyalikh, D.V.; Molodtsova, O.; Preobrajenski, A.B.; Zakharov, A.A.; Hess, C.; Hänke, T.; Büchner, B.; et al. Graphene Synthesis on Cubic SiC/Si Wafers. Perspectives for Mass Production of Graphene-Based Electronic Devices. Nano Lett. 2010, 10, 992–995.
- Avouris, P.; Xia, F. Graphene applications in electronics and photonics. MRS Bull. 2012, 37, 1225–1234.
- Bolotin, K.I.; Sikes, K.J.; Hone, J.; Stormer, H.L.; Kim, P. Temperature-dependent transport in suspended gra-phene. Phys. Rev. Lett. 2008, 101, 096802.
- Bonanni, A.; Pumera, M. Graphene Platform for Hairpin-DNA-Based Impedimetric Genosensing. ACS Nano 2011, 5, 2356–2361.
- Brownson, D.; Kampouris, D.; Banks, C. Graphene electrochemistry: Fundamental concepts through to prominent applications. Chem. Soc. Rev. 2012, 41, 6944–6976.
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568.
- Paredes, J.I.; Villar-Rodil, S.; Fernández-Merino, M.J.; Guardia, L.; Martínez-Alonso, A.; Tascón, J.M.D. Environ-mentally friendly approaches toward the mass production of processable graphene from graphite oxide. J. Mater. Chem. 2011, 21, 298–306.
- Park, S.; An, J.; Piner, R.D.; Jung, I.; Yang, D.; Velamakanni, A.; Nguyen, S.; Ruoff, R.S. Aqueous Suspension and Characterization of Chemically Modified Graphene Sheets. Chem. Mater. 2008, 20, 6592–6594.
- Prasai, D.; Tuberquia, J.C.; Harl, R.R.; Jennings, G.K.; Bolotin, K.I. Graphene: Corrosion-Inhibiting Coating. ACS Nano 2012, 6, 1102–1108.
- Song, W.; Ji, X.; Deng, W.; Chen, Q.; Shen, C.; Banks, C.E. Graphene ultracapacitors: Structural impacts. Phys. Chem. Chem. Phys. 2013, 15, 4799–4803.
- Wang, G.; Yang, J.; Park, J.; Gou, X.; Wang, B.; Liu, H.; Yao, J. Facile synthesis and characterization of graphene nanosheets. J. Phys. Chem. C 2008, 112, 8192–8195.
- Bonaccorso, F.; Sun, Z.; Hasan, T.; Ferrari, A.C. Graphene photonics and optoelectronics. Nat. Photonics 2010, 4, 611–622.
- El-Kady, M.F.; Shao, Y.; Kaner, R.B. Graphene for batteries, supercapacitors and beyond. Nat. Rev. Mater. 2016, 1, 16033.
- Ilnicka, A.; Lukaszewicz, J.P. Graphene-Based Hydrogen Gas Sensors: A Review. Processes 2020, 8, 633.
- Wang, Y.; Shao, Y.; Matson, D.W.; Li, J.; Lin, Y. Nitrogen-Doped Graphene and Its Application in Electrochemical Biosensing. ACS Nano 2010, 4, 1790–1798.
- Lerner, M.B.; Matsunaga, F.; Han, G.H.; Hong, S.J.; Xi, J.; Crook, A.; Perez-Aguilar, J.M.; Park, Y.W.; Saven, J.G.; Liu, R.; et al. Scalable Production of Highly Sensitive Nanosensors Based on Graphene Functionalized with a Designed G Protein-Coupled Receptor. Nano Lett. 2014, 14, 2709–2714.
- Lin, Y.-M.; Dimitrakopoulos, C.; Jenkins, K.A.; Farmer, D.B.; Chiu, H.-Y.; Grill, A.; Avouris, P. 100-GHz Transistors from Wafer-Scale Epitaxial Graphene. Science 2010, 327, 662.
- Wu, Y.; Lin, Y.-M.; Bol, A.; Jenkins, K.A.; Xia, F.; Farmer, D.B.; Zhu, Y.; Avouris, P. High-frequency, scaled graphene transistors on diamond-like carbon. Nature 2011, 472, 74–78.
- Liao, L.; Lin, Y.C.; Bao, M.; Cheng, R.; Bai, J.; Liu, Y.; Qu, Y.; Wang, K.L.; Huang, Y.; Duan, X. High-speed gra-phene transistors with a self-aligned nanowire gate. Nature 2010, 467, 305–308.
- Lalwani, G.; Henslee, A.M.; Farshid, B.; Lin, L.; Kasper, F.; Qin, Y.-X.; Mikos, A.G.; Sitharaman, B. Two-Dimensional Nanostructure-Reinforced Biodegradable Polymeric Nanocomposites for Bone Tissue Engineering. Biomacromolecules 2013, 14, 900–909.
- Blake, P.; Hill, E.W.; Castro Neto, A.H.; Novoselov, K.S.; Jiang, D.; Yang, R.; Booth, T.J.; Geim, A.K. Making gra-phene visible. Appl. Phys. Lett. 2007, 91, 063124.
- Casiraghi, C.; Hartschuh, A.; Lidorikis, E.; Qian, H.; Harutyunyan, H.; Gokus, T.; Novoselov, K.; Ferrari, A.C. Rayleigh Imaging of Graphene and Graphene Layers. Nano Lett. 2007, 7, 2711–2717.
- Mittal, G.; Dhand, V.; Rhee, K.Y.; Park, S.-J.; Lee, W.R. A review on carbon nanotubes and graphene as fillers in reinforced polymer nanocomposites. J. Ind. Eng. Chem. 2014, 21, 11–25.
- Liu, W.W.; Chai, S.-P.; Mohamed, A.R.; Hashim, U. Synthesis and characterization of graphene and carbon nanotubes: A review on the past and recent developments. J. Ind. Eng. Chem. 2014, 20, 1171–1185.
- Rafiee, M.; Lu, W.; Thomas, A.V.; Zandiatashbar, A.; Rafiee, J.; Tour, J.M.; Koratkar, N.A. Graphene Nanoribbon Composites. ACS Nano 2010, 4, 7415–7420.
- Rafiee, M.A.; Rafiee, J.; Wang, Z.; Song, H.; Yu, Z.Z.; Koratkar, N. Enhanced mechanical properties of nanocomposites at low graphene content. ACS Nano 2009, 3, 3884–3890.
- Varela-Rizo, H.; Rodriguez-Pastor, I.; Merino, C.; Martin-Gullon, I. Highly crystalline graphene oxide nano-platelets produced from helical-ribbon carbon nanofibers. Carbon 2010, 48, 3640–3643.
- Viculis, L.M.; Mack, J.J.; Mayer, O.M.; Hahn, H.T.; Kaner, R.B. Intercalation and exfoliation routes to graphite nanoplatelets. J. Mater. Chem. 2005, 15, 974–978.
- Zhao, X.; Zhang, Q.; Chen, D.; Lu, P. Enhanced Mechanical Properties of Graphene-Based Poly(vinyl alcohol) Composites. Macromolecules 2010, 43, 2357–2363.
- Chang, H.; Wu, H. Graphene-based nanocomposites: Preparation, functionalization, and energy and environmental applications. Energy Environ. Sci. 2013, 6, 3483–3507.
- Rasheed, A.; Chae, H.G.; Kumar, S.; Dadmun, M.D. Polymer nanotube nanocomposites: Correlating intermolecular interaction to ultimate properties. Polymer 2006, 47, 4734–4741.
- Burgaz, E. Thermomechanical Analysis of Polymer Nanocomposites BT—Polymer Nanocomposites: Electrical and Thermal Properties; Huang, X., Zhi, C., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 191–242.
- Choudhary, V.; Gupta, A. Polymer/carbon nanotube nanocomposites. In Carbon Nanotubes-Polymer Nanocomposites; IntechOpen: London, UK, 2011; pp. 65–90.
- Du, F.; Scogna, R.C.; Zhou, W.; Brand, S.; Fischer, J.E.; Winey, K.I. Nanotube networks in polymer nanocomposites: Rheology and electrical conductivity. Macromolecules 2004, 37, 9048–9055.
- Li, Q.; Wang, Q. Polymer Nanocomposites for Power Energy Storage BT—Polymer Nanocomposites: Electrical and Thermal Properties; Huang, X., Zhi, C., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 139–163.
- Mclauchlin, A.R.; Thomas, N.L. 13—Biodegradable polymer nanocomposites A2—Gao, Fengge BT—Advances in Polymer Nanocomposites. In Woodhead Publishing Series in Composites Science and Engineering; Woodhead Publishing: Sawston, UK, 2012; pp. 398–430.
- Wakabayashi, K.; Pierre, C.; Dikin, D.A.; Ruoff, R.S.; Ramanathan, T.; Brinson, L.; Torkelson, J.M. Polymer−Graphite Nanocomposites: Effective Dispersion and Major Property Enhancement via Solid-State Shear Pulverization. Macromolecules 2008, 41, 1905–1908.
- Moniruzzaman, M.; Winey, K.I. Polymer Nanocomposites Containing Carbon Nanotubes. Macromolecules 2006, 39, 5194–5205.
- Rangari, V.K.; Dey, S. Synthesis Fabrication and Characterization of Ag/CNT-Polymer Nanocomposites. In Synthesis Techniques for Polymer Nanocomposites; Wiley-VCH Verlag GmbH & Co. KgaA: Hoboken, NJ, USA, 2014; pp. 115–130.
- Marras, S.I.; Kladi, K.P.; Tsivintzelis, I.; Zuburtikudis, I.; Panayiotou, C. Biodegradable polymer nanocomposites: The role of nanoclays on the thermomechanical characteristics and the electrospun fibrous structure. Acta Biomater. 2008, 4, 756–765.
- Wang, Z.; Zhi, C. Thermally Conductive Electrically Insulating Polymer Nanocomposites BT—Polymer Nanocomposites: Electrical and Thermal Properties; Huang, X., Zhi, C., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 281–321.
- Winey, K.I.; Vaia, R.A. Polymer Nanocomposites. MRS Bull. 2007, 32, 314–322.
- Yang, L.; Toh, C.L.; Lu, X. In Situ Preparation of Conducting Polymer Nanocomposites. In Synthesis Techniques for Polymer Nanocomposites; Wiley-VCH Verlag GmbH & Co. KgaA: Hoboken, NJ, USA, 2014; pp. 211–240.
- Zope, I.S.; Dasari, A. High-Temperature-Resistant Polymer Nanocomposites. In Functional and Physical Properties of Polymer Nanocomposites; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 183–201.
- Jancar, J.; Douglas, J.; Starr, F.; Kumar, S.; Cassagnau, P.; Lesser, A.; Sternstein, S.; Buehler, M. Current issues in research on structure–property relationships in polymer nanocomposites. Polymer 2010, 51, 3321–3343.
- Arash, B.; Wang, Q.; Varadan, V.K. Mechanical properties of carbon nanotube/polymer composites. Sci. Rep. 2014, 4, 6479.
- Crosby, A.J.; Lee, J.Y. Polymer Nanocomposites: The “Nano” Effect on Mechanical Properties. Polym. Rev. 2007, 47, 217–229.
- Nguyen, B.H.; Nguyen, V.H. Promising applications of graphene and graphene-based nanostructures. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 023002.
- Tanaka, T.; Montanari, G.C.; Mulhaupt, R. Polymer nanocomposites as dielectrics and electrical insulation-perspectives for processing technologies, material characterization and future applications. IEEE Trans. Dielectr. Electr. Insul. 2004, 11, 763–784.
- Park, J.K.; Do, I.-H.; Askeland, P.; Drzal, L.T. Electrodeposition of exfoliated graphite nanoplatelets onto carbon fibers and properties of their epoxy composites. Compos. Sci. Technol. 2008, 68, 1734–1741.
- Paul, D.; Robeson, L. Polymer nanotechnology: Nanocomposites. Polymer 2008, 49, 3187–3204.
- Alexandre, M.; Dubois, P. Polymer-layered silicate nanocomposites: Preparation, properties and uses of a new class of materials. Mater. Sci. Eng. R Rep. 2000, 28, 1–63.
- Unalan, I.U.; Cerri, G.; Marcuzzo, E.; Cozzolino, C.A.; Farris, S. Nanocomposite films and coatings using inorganic nanobuilding blocks (NBB): Current applications and future opportunities in the food packaging sector. RSC Adv. 2014, 4, 29393–29428.
- Costantino, A.; Pettarin, V.; Viana, J.; Pontes, A.; Pouzada, A.; Frontini, P. Microstructure of PP/clay nanocomposites produced by shear induced injection moulding. Procedia Mater. Sci. 2012, 1, 34–43.
- Khvan, S.; Kim, J.; Lee, S.-S. Fabrication of pre-exfoliated clay masterbatch via exfoliation-adsorption of polystyrene nanobeads. Macromol. Res. 2007, 15, 51–58.
- Li, B.; Hu, Y.; Liu, J.; Chen, Z.; Fan, W. Preparation of poly (methyl methacrylate)/LDH nanocomposite by exfoliation-adsorption process. Colloid Polym. Sci. 2003, 281, 998–1001.
- Vazquez, A.; Cyras, V.P.; Alvarez, V.A.; Moran, J.I. Starch/clay nano-biocomposites. In Environmental Silicate Nano-Biocomposites; Springer: London, UK, 2012; pp. 287–321.
- Uhl, F.M.; Wilkie, C.A. Polystyrene/graphite nanocomposites: Effect on thermal stability. Polym. Degrad. Stab. 2002, 76, 111–122.
- Zhang, H.-B.; Zheng, W.-G.; Yan, Q.; Jiang, Z.-G.; Yu, Z.-Z. The effect of surface chemistry of graphene on rheological and electrical properties of polymethylmethacrylate composites. Carbon 2012, 50, 5117–5125.
- Kim, H.; Miura, Y.; Macosko, C.W. Graphene/Polyurethane Nanocomposites for Improved Gas Barrier and Electrical Conductivity. Chem. Mater. 2010, 22, 3441–3450.
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior Thermal Conductivity of Single-Layer Graphene. Nano Lett. 2008, 8, 902–907.
- Huang, P.; Ruiz-Vargas, C.S.; van der Zande, A.; Whitney, W.S.; Levendorf, M.P.; Kevek, J.W.; Garg, S.; Alden, J.S.; Hustedt, C.J.; Zhu, Y.; et al. Grains and grain boundaries in single-layer graphene atomic patchwork quilts. Nature 2011, 469, 389–392.
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.-S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Kim, H.R.; Song, Y.I.; et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010, 5, 574–578.
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-Area Synthesis of High-Quality and Uniform Graphene Films on Copper Foils. Science 2009, 324, 1312–1314.
- Zhang, T.; Li, X.; Gao, H. Defects controlled wrinkling and topological design in graphene. J. Mech. Phys. Solids 2014, 67, 2–13.
- Yazyev, O.; Louie, S.G. Electronic transport in polycrystalline graphene. Nat. Mater. 2010, 9, 806–809.
- Yazyev, O.; Louie, S.G. Topological defects in graphene: Dislocations and grain boundaries. Phys. Rev. B 2010, 81, 195420.
- Lusk, M.T.; Carr, L. Nanoengineering Defect Structures on Graphene. Phys. Rev. Lett. 2008, 100, 175503.
- Ali, G.; Mehmood, A.; Ha, H.Y.; Kim, J.; Chung, K.Y. Reduced graphene oxide as a stable and high-capacity cathode material for Na-ion batteries. Sci. Rep. 2017, 7, 40910.
- Sharma, B.; Kim, J.-S. MEMS based highly sensitive dual FET gas sensor using graphene decorated Pd-Ag alloy nanoparticles for H2 detection. Sci. Rep. 2018, 8, 5902.
- Georgakilas, V. Functionalization of Graphene by other Carbon Nanostructures. In Functionalization of Graphene; Wiley-VCH Verlag GmbH & Co. KgaA: Hoboken, NJ, USA, 2014; pp. 255–282.
