Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
LAT1-4F2hc complex is an important amino acid transporter. It mainly transports specific amino acids through the cell membrane, provides nutrition for cells, and participates in a variety of metabolic pathways. LAT1 plays a role in transporting essential amino acids including leucine, which regulates the mTOR signaling pathway.
- LAT1-4F2hc
- cancer
- Urology
- Prostate Cancer
- Renal Cancer
- Bladder Cancer
1. Introduction
Continuous proliferative signaling is the main feature of malignant tumors [1]. These signals trigger tumor cells to divide, causing tumor cells to grow rapidly in an uncontrollable way. Among all of these nutrients, Eagle discovered in 1955 that essential amino acids (EAA) were required for cell growth in vitro [2]. Later, studies found that the uptake of EAA in malignant tumor cells was higher than in normal tissues [3][4][5]. After being delivered into the cells, these amino acids were utilized to make proteins, nucleic acids, lipids, and ATP. Cancer cells have higher up-regulated transporters that facilitate the entrance of exogenous amino acids into cells, compared to normal cells, and the steady acquisition of amino acids by cancer cells is important for cancer growth [6]. HATs (heteromeric amino acid transporters) are a special type of solute transporter. They are made up of two subunits, one heavy and one light, that are linked by a conserved disulfide bond [7]. The heavy subunit is a member of the SLC3 family, whereas the light subunit belongs to the SLC7 family.
The SLC3 family now includes two glycoproteins (rBAT (SLC3A1)) and 4F2hc (SLC3A2, also known as CD98) [7]. Heavy subunits of the SLC3 family, such as 4F2hc, were discovered in 1998 and are necessary for the proper trafficking of the heterodimer to the plasma membrane [8].
Regarding the SLC7 family, Kanai first isolated a cDNA from rat C6 glioma cells through expression cloning in 1998. The cDNA encodes a new Na +-independent neutral amino acid transporter called LAT1 [9]. In 1999, Kanai’s team further isolated a cDNA from the rat small intestine, which encodes another transporter called LAT2 [10]. The former two proteins belong to the solute carrier family 7 (SLC7). After that, LAT3 [11] and LAT4 [12] were gradually discovered. These two belong to the SLC43 family. The L-type amino acid transporter, which consists of all former four subunits (LAT1-4), is an important pathway for EAA to enter the cell. Subsequently, Wang found that (18) F-labeled fluoroalkyl phenylalanine derivatives as PET tracers were more likely to bind to LAT1 in tumors, and the specific accumulation of this tracer in tumor cells suggested that LAT1 was expressed in a large number of malignant tumors, thus preliminarily revealing the close relationship between LAT1 and malignant tumors [13].
2. LAT1/4F2hc and Human Diseases (Pain & Inflammation)
Existing studies have found that LAT1-4F2hc complex is widely associated with human diseases, such as inflammation, pain, hypoxia, and tumors [14][15][16].
Inhibition of LAT1 eliminated mTORC1 activation, plasmablast differentiation, and CpG (toll-like receptor TLR9 ligand)-stimulated B cell production of IgG and inflammatory cytokines. The influx of L-leucine through LAT1 regulates the activity of mTORC1 and the immune response of human B cells [15][16]. Among the most common nociceptive pathways, LAT1 may be a feasible new target for pain. LAT1 expression and regulation link it to key cell types and pathways related to pain. LAT1 regulates the Wnt/frizzled/β-catenin signal transduction pathway. The LAT1-4F2hc complex may also be involved in pain pathways related to T cells and B cells. The expression of LAT1 induces the activation of the mammalian target of rapamycin (mTOR) signal axis, which is related to inflammation and neuropathic pain. Similarly, hypoxia and tumors can induce the activation of hypoxia-inducible factor 2α, which not only promotes the expression of LAT1 but also promotes the activation of mTORC1 [14]. As the common node of the T cell, B cell, and mTOR pathway, LAT1-4F2hc plays a vital role in human diseases. It has also received increasing attention as an important target for autoimmune diseases, chronic pain diseases, and tumors.
3. LAT1/4F2hc and Tumors
Many tumor cells lines [17][18][19] and human malignancies, such as breast, prostate, lung, colorectal, and gliomas [20][21][22][23][24][25], have high levels of LAT1 expression. In these tumors, LAT1 plays an important role in growth and survival. RNA interference (RNAi) [22][26][27][28][29] and genetic disruption by zinc fingers nucleases-mediated [30] LAT1-knockout in cancer cells caused that leucine absorption and cell proliferation were both inhibited. As a result, LAT1 is being evaluated as a potential therapeutic target for reducing cancer cell growth and proliferation [31][32].
Similarly, in human neoplasms such as prostate cancer, gastric cancer, lung pleomorphic carcinoma, and neuroendocrine carcinoma, 4F2hc expression is upregulated [33][34][19][35]. Increased 4F2hc expression is linked to a worse chance of survival, cell proliferation, and metastasis [36]. Since 4F2hc binds with LAT1 on the membranous surface of cancer cells, these results are not difficult to understand.
The LAT1-4F2hc complex is also closely related to tumor glutamine metabolism. The amount of glutamine required by cancer cells exceeds the supply produced by endogenous synthesis, resulting in the up-regulation of glutamine metabolism in many carcinogenic changes. LAT1-4F2hc complex controls the flux of glutamine and other amino acids involved in glutaminolysis and glutamine-regulated homeostasis [37]. LAT1-4F2hc complex exchanges Gln for leucine and other amino acids, which can lead to mTOR activation.
By influencing the mammalian target protein of rapamycin complex 1 (mTORC1), the amino acid leucine has been demonstrated to increase protein synthesis and accelerate cell development, whereas LAT1 has been linked to mTORC1 signaling and, as a result, cancer progression [6][38].
In cancer cells, however, LAT1 not only boosts mTORC1 activity but also enhances MYC and EZH2 signaling. Through the AKT, MAPK, and cell-cycle related P21 and P27 signal pathways, 4F2hc has been demonstrated to affect cancer cell proliferation. The expression of 4F2hc and LAT1 is reportedly codependent, and the downregulation of either subunit destabilizes the partner [8]. (Figure 1, Table 1).
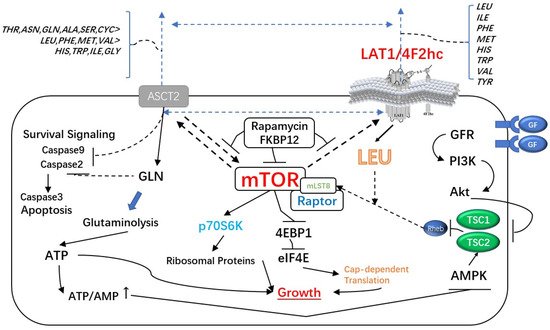
Figure 1. The Major Signaling Pathways Affected by LAT1-4F2hc Complex: The LAT1-4F2HC complex not only enhances mTORC1 activity but also enhances MYC and EZH2 signaling pathways. Moreover, it can affect the proliferation of cancer cells through AKT, MAPK and cell cycle-related P21 and P27 signaling pathways.
Table 1. LAT1-4F2hc and Common Tumors.
| Cancer Types | Cell Lines | Downstream Effects of LAT1/4F2hc | Other Related Factors | References | |
|---|---|---|---|---|---|
| LAT1-4F2hc Complex | NSCLC | A549, H1299 |
Mice with smaller tumors, lower leucine absorption, lower mTORC1 activity, amino acid stress, lower proliferation, and lower EZH2 expression and activity | Ki-67, VEGF, CD31, CD34, HIF-1a, mTOR, ASCT2 |
[34][30][39][40][41][42][43][44] |
| Gastric cancer | SGC-7901, MKN-45, MGC-803, CRL-5974 |
Deceases in proliferation, migration and invasion | Ki-67 | [45][46][47][48][49][50][51] | |
| Pancreatic cancer | MIA, Paca-2 |
Reductions in mTORC1 activity, decreases in proliferation and angiogenesis | Ki-67, VEGF, c-Myc, CD147 |
[28][52][53][54][55][56] | |
| Biliary tract cancer | KKU-M055, KKU-M213 | JPH203 first in human phase I clinical trial. Well-tolerated. | Ki-67 | [57][58][59][60][61][62][63] | |
| Ovarian cancer | SKOV3, IGROV1, A2780, OVCAR-3 |
Decreases in proliferation | ASCT2, SN2, p70S6K, LAT2 |
[64][65][66][67] | |
| Breast cancer & TNBC | MCF-7, ZR-75, MDA-MB-232 |
Decreases in proliferation | ADS, HER2, TN, Ki-67, ER, PgR |
[23][68][69][70][71][72] |
4. Inhibitors of LAT1/4F2hc and Targeted Therapy
Due to its own transport characteristics of the SLC family, the LAT1-4F2hc complex often plays a key role in drug absorption, distribution and toxicity by mediating drug transmembrane transport [37]. However, only a small number of SLCs have been locked by drugs or chemical probes till now. Three main factors hinder the development of new chemical entities that can regulate SLC activity. First, most studies on this super population are relatively insufficient, and the biological functions or substrates of many SLCs are still unclear. Second, there is a lack of high-quality biological tools, specific, and reliable reagents and special databases. Finally, the number of functional analyses required to study such diverse objectives is still limited [73]. It is reported radioligand uptake assays have been widely employed to study LAT1 [74], but the radioligand uptake assays cannot distinguish inhibitors from substrates. The LAT1-4F2hc complex is overexpressed in many cancer cells and is thought to be a viable anticancer therapeutic target since inhibiting it reduces cancer cell viability dramatically.
BCH and JPH203 are LAT1-4F2hc complex inhibitors that have been studied extensively. BCH is a non-metabolic leucine analogue. In 2006, Baniasadi [75] found that BCH has an impact on the expression of many genes involved in cell survival and physiological activity. These data help to understand the intracellular signal transduction of cell growth inhibition induced by LAT1 inhibitors and can be used as a candidate for anticancer drug therapy [75]. Later studies proposed the use of N-butyl-N- (4-hydroxybutyl) nitrosamine (BBN) treatment to induce high expression of LAT1/4F2hc in rat bladder cancer cells [76] and proposed some directions for anti-LAT1/4F2hc drugs. JPH203 was discovered by Oda in 2010 and was originally known as KYT-0353 [77]. JHP203 is a highly selective LAT1 inhibitor produced by synthetic chemistry and in vitro screening based on triiodothyronine (T3). JPH203 showed excellent selective inhibition of LAT1 and showed potential as a novel antitumor agent. JPH203 interferes with constitutive activation of mTORC1 and Akt, reduces c-MyC expression, and triggers a folding protein response mediated by CHOP transcription factors associated with cell death [78]. Since then, several studies have confirmed that JPH203 has an impressive inhibitory effect on the growth of common tumor cells, such as colon cancer [77][79], gastric carcinoma [46], medulloblastoma [80], osteosarcoma [81], thyroid cancer [82][83], endocrine-resistant breast cancer [84], pituitary tumor [85], head and neck cancer cells [86], and T-cell Acute lymphoblastic leukemia (T-ALL)/lymphoma (T-LL) cells [78], etc.
In terms of urinary tumors, Maimaiti [87] found that in bladder cancer cells JPH203 inhibits the absorption of leucine by >90%. JPH203 inhibits the phosphorylation of MAPK/Erk, AKT, p70S6K, and 4EBP-1. JPH203 inhibits IGF-mediated igfb5 expression and AKT phosphorylation [87].
In the area of RCC, Higuchi [88] has tested the effects of JPH203 on RCC-derived Caki-1 and ACHN cells. JPH203 suppressed the proliferation of various cell types in a dose-dependent manner. According to the findings, the migration and invasion operations were stifled by JPH203 [88].
In the area of PCa, Otsuki [89] found that LAT1 was primarily expressed in DU145 and PC-3 cells. BCH or JPH203 inhibited leucine uptake and cell proliferation in a dose-dependent manner [89]. A Phase I clinical study found that JPH203 was well-tolerated and provided promising activity against biliary tract cancer [90]. The authors are currently planning Phase I and II study of JPH203 in CRPC [90].
These studies also show the potential of JPH203 for the treatment of urological cancers.
In 2021, Yan [91] synthesized three LAT1 inhibitors, JX-075, JX-078, and JX-119, and used cryo-EM to solve the inhibitors’ complex structures with the LAT1-4F2hc complex. They also solved the LAT1-4F2hc complex coupled with Diiodo-Tyr’s cryo-EM structure. LAT1 is found in an outward-occluded conformation in all the combinations of these complexes. These structures might reflect two distinct inhibitory processes, giving significant information for medication development in the future [91].
Of particular interest is the first Phase I clinical trial of JPH203 [90]. Although several studies have demonstrated that JPH203 can inhibit leucine uptake by tumor cells and show concentration-dependent cytotoxicity in vitro or good results in transplanted tumor models, Phase I clinical trial in humans is a milestone. Okano assessed dose-limiting toxicity in the first cycle using the 3 + 3 design. Seventeen Japanese patients with advanced solid tumors were enrolled and treated daily with JPH203 intravenously for 7 days. The maximum safe tolerated dose of JPH203 was defined as 60 mg/m2. The suitable RP2D is 25 mg/m2. Partial response was observed in one biliary tract cancer (BTC) patient at 12 mg/m2, and disease control was achieved in three of the six BTC patients at both the 12 mg/m2 and 25 mg/m2 levels. The disease control rate of BTC was 60%. The JPH203 molecule is predominantly metabolized into Nac-JPH203 by N-acetyltransferase 2 in liver cells [92]. Patients’ N-acetyltransferase 2 phenotype (rapid/non-rapid) was found to predict the safety and efficacy of JPH203. A lower Nac-JPH203/JPH203 ratio is critical for maximizing the anti-tumor effect of JPH203 [90].
Of course, there are still some deficiencies and limitations in the study of urinary tumors and LAT1-4F2hc complexes mentioned above.
In BBN-induced bladder cancer, LAT1-4F2hc was not expressed by porous endothelial cells. Whether LAT1-4F2hc expression depends on endothelial cell structure is unclear. Fenestration of microvascular endothelial cells is not a stable event, because endothelial cells with fenestration in BBN-induced rat bladder cancer were transformed into endothelial cells without fenestration 5 min after injection of VEGF inhibitor, and fenestration recovered 30 min later [76]. The molecular mechanisms of amino acid transport in normal and tumor microvascular endothelial cells need further study. However, the LAT1-4F2hc complex is closely related to angiogenesis [19][53][63][76][93][94][95][96][97]. This makes it possible for the LAT1-4F2hc complex to improve the effectiveness of cancer immunotherapy by improving immune vascular crosstalk [98].
In prostate cancer-related experiments, although downregulation of LAT1 and LAT3 in tumor cells inhibits the growth of prostate cancer cells, it remains to be determined what other mechanisms of prostate cancer resistance can be triggered by targeting LAT1 (such as activation of ATF4).
Most of the studies were conducted in vitro, not in vivo. Although the phase I clinical trial of JPH203 against biliary tract cancer has achieved good results, the clinical trial has not yet involved any urinary tumors. In addition, the number of patients included in some studies is relatively small, or the follow-up time is not long, and the prognostic impact of LAT1 inhibition on tumor patients with different stages has not been thoroughly solved. Most of the specimens studied are in vitro tumor specimens after surgery, and the expression of LAT1-4F2hc in early tumors and its influence on tumors are also a key link that needs to be studied.
Finally, targeted therapy of LAT1-4F2hc does not directly kill cancer cells, but blocks amino acid transport, resulting in loss of nutritional basis and self-apoptosis of cancer cells. This has led some investigators to suggest that targeting LAT1-4F2hc is more suitable for slow-progressing tumors. Therefore, further studies are needed to obtain more evidence that LAT1-4F2HC therapy is also suitable for highly aggressive and rapidly progressing tumors.
This entry is adapted from the peer-reviewed paper 10.3390/cancers14010229
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674.
- Eagle, H. Nutrition needs of mammalian cells in tissue culture. Science 1955, 122, 501–514.
- Qi, W.; Guan, Q.; Sun, T.; Cao, Y.; Zhang, L.; Guo, Y. Improving detection sensitivity of amino acids in thyroid tissues by using phthalic acid as a mobile phase additive in hydrophilic interaction chromatography-electrospray ionization-tandem mass spectrometry. Anal. Chim. Acta 2015, 870, 75–82.
- Kirikae, M.; Diksic, M.; Yamamoto, Y.L. Quantitative measurements of regional glucose utilization and rate of valine incorporation into proteins by double-tracer autoradiography in the rat brain tumor model. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 1989, 9, 87–95.
- Wang, L.B.; Shen, J.G.; Zhang, S.Z.; Ding, K.F.; Zheng, S. Amino acid uptake in arterio-venous serum of normal and cancerous colon tissues. World J. Gastroenterol. 2004, 10, 1297–1300.
- Wang, Q.; Holst, J. L-type amino acid transport and cancer: Targeting the mTORC1 pathway to inhibit neoplasia. Am. J. Cancer Res. 2015, 5, 1281–1294.
- Fotiadis, D.; Kanai, Y.; Palacin, M. The SLC3 and SLC7 families of amino acid transporters. Mol. Asp. Med. 2013, 34, 139–158.
- Mastroberardino, L.; Spindler, B.; Pfeiffer, R.; Skelly, P.J.; Loffing, J.; Shoemaker, C.B.; Verrey, F. Amino-acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature 1998, 395, 288–291.
- Kanai, Y.; Segawa, H.; Miyamoto, K.; Uchino, H.; Takeda, E.; Endou, H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98). J. Biol. Chem. 1998, 273, 23629–23632.
- Segawa, H.; Fukasawa, Y.; Miyamoto, K.; Takeda, E.; Endou, H.; Kanai, Y. Identification and functional characterization of a Na+-independent neutral amino acid transporter with broad substrate selectivity. J. Biol. Chem. 1999, 274, 19745–19751.
- Babu, E.; Kanai, Y.; Chairoungdua, A.; Kim, D.K.; Iribe, Y.; Tangtrongsup, S.; Jutabha, P.; Li, Y.; Ahmed, N.; Sakamoto, S.; et al. Identification of a novel system L amino acid transporter structurally distinct from heterodimeric amino acid transporters. J. Biol. Chem. 2003, 278, 43838–43845.
- Bodoy, S.; Martin, L.; Zorzano, A.; Palacin, M.; Estevez, R.; Bertran, J. Identification of LAT4, a novel amino acid transporter with system L activity. J. Biol. Chem. 2005, 280, 12002–12011.
- Wang, L.; Qu, W.; Lieberman, B.P.; Plossl, K.; Kung, H.F. Synthesis, uptake mechanism characterization and biological evaluation of (18)F labeled fluoroalkyl phenylalanine analogs as potential PET imaging agents. Nucl. Med. Biol. 2011, 38, 53–62.
- Alles, S.R.A.; Gomez, K.; Moutal, A.; Khanna, R. Putative roles of SLC7A5 (LAT1) transporter in pain. Neurobiol. Pain 2020, 8, 100050.
- Torigoe, M.; Maeshima, K.; Ozaki, T.; Omura, Y.; Gotoh, K.; Tanaka, Y.; Ishii, K.; Shibata, H. l-Leucine influx through Slc7a5 regulates inflammatory responses of human B cells via mammalian target of rapamycin complex 1 signaling. Mod. Rheumatol. 2019, 29, 885–891.
- Behzadi, P.; Garcia-Perdomo, H.A.; Karpinski, T.M. Toll-Like Receptors: General Molecular and Structural Biology. J. Immunol. Res. 2021, 2021, 9914854.
- Fuchs, B.C.; Bode, B.P. Amino acid transporters ASCT2 and LAT1 in cancer: Partners in crime? Semin. Cancer Biol. 2005, 15, 254–266.
- Kobayashi, K.; Ohnishi, A.; Promsuk, J.; Shimizu, S.; Kanai, Y.; Shiokawa, Y.; Nagane, M. Enhanced tumor growth elicited by L-type amino acid transporter 1 in human malignant glioma cells. Neurosurgery 2008, 62, 493–503, discussion 494–503.
- Kaira, K.; Oriuchi, N.; Imai, H.; Shimizu, K.; Yanagitani, N.; Sunaga, N.; Hisada, T.; Tanaka, S.; Ishizuka, T.; Kanai, Y.; et al. l-type amino acid transporter 1 and CD98 expression in primary and metastatic sites of human neoplasms. Cancer Sci. 2008, 99, 2380–2386.
- Betsunoh, H.; Fukuda, T.; Anzai, N.; Nishihara, D.; Mizuno, T.; Yuki, H.; Masuda, A.; Yamaguchi, Y.; Abe, H.; Yashi, M.; et al. Increased expression of system large amino acid transporter (LAT)-1 mRNA is associated with invasive potential and unfavorable prognosis of human clear cell renal cell carcinoma. BMC Cancer 2013, 13, 509.
- Ebara, T.; Kaira, K.; Saito, J.; Shioya, M.; Asao, T.; Takahashi, T.; Sakurai, H.; Kanai, Y.; Kuwano, H.; Nakano, T. L-type amino-acid transporter 1 expression predicts the response to preoperative hyperthermo-chemoradiotherapy for advanced rectal cancer. Anticancer Res. 2010, 30, 4223–4227.
- Xu, M.; Sakamoto, S.; Matsushima, J.; Kimura, T.; Ueda, T.; Mizokami, A.; Kanai, Y.; Ichikawa, T. Up-Regulation of LAT1 during Antiandrogen Therapy Contributes to Progression in Prostate Cancer Cells. J. Urol. 2016, 195, 1588–1597.
- Furuya, M.; Horiguchi, J.; Nakajima, H.; Kanai, Y.; Oyama, T. Correlation of L-type amino acid transporter 1 and CD98 expression with triple negative breast cancer prognosis. Cancer Sci. 2012, 103, 382–389.
- Nawashiro, H.; Otani, N.; Shinomiya, N.; Fukui, S.; Ooigawa, H.; Shima, K.; Matsuo, H.; Kanai, Y.; Endou, H. L-type amino acid transporter 1 as a potential molecular target in human astrocytic tumors. Int. J. Cancer 2006, 119, 484–492.
- Sakata, T.; Ferdous, G.; Tsuruta, T.; Satoh, T.; Baba, S.; Muto, T.; Ueno, A.; Kanai, Y.; Endou, H.; Okayasu, I. L-type amino-acid transporter 1 as a novel biomarker for high-grade malignancy in prostate cancer. Pathol. Int. 2009, 59, 7–18.
- Kim, C.H.; Park, K.J.; Park, J.R.; Kanai, Y.; Endou, H.; Park, J.C.; Kim, D.K. The RNA interference of amino acid transporter LAT1 inhibits the growth of KB human oral cancer cells. Anticancer Res. 2006, 26, 2943–2948.
- Marshall, A.D.; van Geldermalsen, M.; Otte, N.J.; Anderson, L.A.; Lum, T.; Vellozzi, M.A.; Zhang, B.K.; Thoeng, A.; Wang, Q.; Rasko, J.E.; et al. LAT1 is a putative therapeutic target in endometrioid endometrial carcinoma. Int. J. Cancer 2016, 139, 2529–2539.
- Hayashi, K.; Jutabha, P.; Endou, H.; Anzai, N. c-Myc is crucial for the expression of LAT1 in MIA Paca-2 human pancreatic cancer cells. Oncol. Rep. 2012, 28, 862–866.
- Liang, Z.; Cho, H.T.; Williams, L.; Zhu, A.; Liang, K.; Huang, K.; Wu, H.; Jiang, C.; Hong, S.; Crowe, R.; et al. Potential Biomarker of L-type Amino Acid Transporter 1 in Breast Cancer Progression. Nucl. Med. Mol. Imaging 2011, 45, 93–102.
- Takeuchi, K.; Ogata, S.; Nakanishi, K.; Ozeki, Y.; Hiroi, S.; Tominaga, S.; Aida, S.; Matsuo, H.; Sakata, T.; Kawai, T. LAT1 expression in non-small-cell lung carcinomas: Analyses by semiquantitative reverse transcription-PCR (237 cases) and immunohistochemistry (295 cases). Lung Cancer 2010, 68, 58–65.
- Cormerais, Y.; Giuliano, S.; LeFloch, R.; Front, B.; Durivault, J.; Tambutté, E.; Massard, P.A.; de la Ballina, L.R.; Endou, H.; Wempe, M.F.; et al. Genetic Disruption of the Multifunctional CD98/LAT1 Complex Demonstrates the Key Role of Essential Amino Acid Transport in the Control of mTORC1 and Tumor Growth. Cancer Res. 2016, 76, 4481–4492.
- Nakanishi, T.; Tamai, I. Solute carrier transporters as targets for drug delivery and pharmacological intervention for chemotherapy. J. Pharm. Sci. 2011, 100, 3731–3750.
- Maimaiti, M.; Sakamoto, S.; Sugiura, M.; Kanesaka, M.; Fujimoto, A.; Matsusaka, K.; Xu, M.; Ando, K.; Saito, S.; Wakai, K.; et al. The heavy chain of 4F2 antigen promote prostate cancer progression via SKP-2. Sci. Rep. 2021, 11, 11478.
- Kaira, K.; Kawashima, O.; Endoh, H.; Imaizumi, K.; Goto, Y.; Kamiyoshihara, M.; Sugano, M.; Yamamoto, R.; Osaki, T.; Tanaka, S.; et al. Expression of amino acid transporter (LAT1 and 4F2hc) in pulmonary pleomorphic carcinoma. Hum. Pathol. 2019, 84, 142–149.
- Satoh, T.; Kaira, K.; Takahashi, K.; Takahashi, N.; Kanai, Y.; Asao, T.; Horiguchi, J.; Oyama, T. Prognostic Significance of the Expression of CD98 (4F2hc) in Gastric Cancer. Anticancer Res. 2017, 37, 631–636.
- Toyoda, M.; Kaira, K.; Shino, M.; Sakakura, K.; Takahashi, K.; Takayasu, Y.; Tominaga, H.; Oriuchi, N.; Nikkuni, O.; Suzuki, M.; et al. CD98 as a novel prognostic indicator for patients with stage III/IV hypopharyngeal squamous cell carcinoma. Head Neck 2015, 37, 1569–1574.
- Wang, W.W.; Gallo, L.; Jadhav, A.; Hawkins, R.; Parker, C.G. The Druggability of Solute Carriers. J. Med. Chem. 2020, 63, 3834–3867.
- Wang, Q.; Tiffen, J.; Bailey, C.G.; Lehman, M.L.; Ritchie, W.; Fazli, L.; Metierre, C.; Feng, Y.J.; Li, E.; Gleave, M.; et al. Targeting amino acid transport in metastatic castration-resistant prostate cancer: Effects on cell cycle, cell growth, and tumor development. J. Natl. Cancer Inst. 2013, 105, 1463–1473.
- Rajasinghe, L.D.; Hutchings, M.; Gupta, S.V. Delta-Tocotrienol Modulates Glutamine Dependence by Inhibiting ASCT2 and LAT1 Transporters in Non-Small Cell Lung Cancer (NSCLC) Cells: A Metabolomic Approach. Metabolites 2019, 9, 50.
- Kaira, K.; Takahashi, T.; Murakami, H.; Shukuya, T.; Kenmotsu, H.; Naito, T.; Oriuchi, N.; Kanai, Y.; Endo, M.; Kondo, H.; et al. Relationship between LAT1 expression and response to platinum-based chemotherapy in non-small cell lung cancer patients with postoperative recurrence. Anticancer Res. 2011, 31, 3775–3782.
- Kaira, K.; Oriuchi, N.; Takahashi, T.; Nakagawa, K.; Ohde, Y.; Okumura, T.; Murakami, H.; Shukuya, T.; Kenmotsu, H.; Naito, T.; et al. LAT1 expression is closely associated with hypoxic markers and mTOR in resected non-small cell lung cancer. Am. J. Transl. Res. 2011, 3, 468–478.
- Kaira, K.; Oriuchi, N.; Imai, H.; Shimizu, K.; Yanagitani, N.; Sunaga, N.; Hisada, T.; Kawashima, O.; Kamide, Y.; Ishizuka, T.; et al. Prognostic significance of L-type amino acid transporter 1 (LAT1) and 4F2 heavy chain (CD98) expression in surgically resectable stage III non-small cell lung cancer. Exp. Ther. Med. 2010, 1, 799–808.
- Kaira, K.; Oriuchi, N.; Imai, H.; Shimizu, K.; Yanagitani, N.; Sunaga, N.; Hisada, T.; Kawashima, O.; Kamide, Y.; Ishizuka, T.; et al. CD98 expression is associated with poor prognosis in resected non-small-cell lung cancer with lymph node metastases. Ann. Surg. Oncol. 2009, 16, 3473–3481.
- Dann, S.G.; Ryskin, M.; Barsotti, A.M.; Golas, J.; Shi, C.; Miranda, M.; Hosselet, C.; Lemon, L.; Lucas, J.; Karnoub, M.; et al. Reciprocal regulation of amino acid import and epigenetic state through Lat1 and EZH2. EMBO J. 2015, 34, 1773–1785.
- Horita, Y.; Kaira, K.; Kawasaki, T.; Mihara, Y.; Sakuramoto, S.; Yamaguchi, S.; Okamoto, K.; Ryozawa, S.; Kanai, Y.; Yasuda, M.; et al. Expression of LAT1 and 4F2hc in Gastroenteropancreatic Neuroendocrine Neoplasms. In Vivo 2021, 35, 2425–2432.
- Muto, Y.; Furihata, T.; Kaneko, M.; Higuchi, K.; Okunushi, K.; Morio, H.; Reien, Y.; Uesato, M.; Matsubara, H.; Anzai, N. Different Response Profiles of Gastrointestinal Cancer Cells to an L-Type Amino Acid Transporter Inhibitor, JPH203. Anticancer Res. 2019, 39, 159–165.
- Ding, K.; Tan, S.; Huang, X.; Wang, X.; Li, X.; Fan, R.; Zhu, Y.; Lobie, P.E.; Wang, W.; Wu, Z. GSE1 predicts poor survival outcome in gastric cancer patients by SLC7A5 enhancement of tumor growth and metastasis. J. Biol. Chem. 2018, 293, 3949–3964.
- Wang, J.; Fei, X.; Wu, W.; Chen, X.; Su, L.; Zhu, Z.; Zhou, Y. SLC7A5 Functions as a Downstream Target Modulated by CRKL in Metastasis Process of Gastric Cancer SGC-7901 Cells. PLoS ONE 2016, 11, e0166147.
- Ichinoe, M.; Yanagisawa, N.; Mikami, T.; Hana, K.; Nakada, N.; Endou, H.; Okayasu, I.; Murakumo, Y. L-Type amino acid transporter 1 (LAT1) expression in lymph node metastasis of gastric carcinoma: Its correlation with size of metastatic lesion and Ki-67 labeling. Pathol. Res. Pract. 2015, 211, 533–538.
- Shi, L.; Luo, W.; Huang, W.; Huang, S.; Huang, G. Downregulation of L-type amino acid transporter 1 expression inhibits the growth, migration and invasion of gastric cancer cells. Oncol. Lett. 2013, 6, 106–112.
- Wang, J.; Chen, X.; Su, L.; Li, P.; Liu, B.; Zhu, Z. LAT-1 functions as a promotor in gastric cancer associated with clinicopathologic features. Biomed. Pharmacother. 2013, 67, 693–699.
- Sampedro-Núñez, M.; Bouthelier, A.; Serrano-Somavilla, A.; Martínez-Hernández, R.; Adrados, M.; Martín-Pérez, E.; Muñoz de Nova, J.L.; Cameselle-Teijeiro, J.M.; Blanco-Carrera, C.; Cabezas-Agricola, J.M.; et al. LAT-1 and GLUT-1 Carrier Expression and Its Prognostic Value in Gastroenteropancreatic Neuroendocrine Tumors. Cancers 2020, 12, 2968.
- Altan, B.; Kaira, K.; Watanabe, A.; Kubo, N.; Bao, P.; Dolgormaa, G.; Bilguun, E.O.; Araki, K.; Kanai, Y.; Yokobori, T.; et al. Relationship between LAT1 expression and resistance to chemotherapy in pancreatic ductal adenocarcinoma. Cancer Chemother. Pharm. 2018, 81, 141–153.
- Kaira, K.; Arakawa, K.; Shimizu, K.; Oriuchi, N.; Nagamori, S.; Kanai, Y.; Oyama, T.; Takeyoshi, I. Relationship between CD147 and expression of amino acid transporters (LAT1 and ASCT2) in patients with pancreatic cancer. Am. J. Transl. Res. 2015, 7, 356–363.
- Yanagisawa, N.; Ichinoe, M.; Mikami, T.; Nakada, N.; Hana, K.; Koizumi, W.; Endou, H.; Okayasu, I. High expression of L-type amino acid transporter 1 (LAT1) predicts poor prognosis in pancreatic ductal adenocarcinomas. J. Clin. Pathol. 2012, 65, 1019–1023.
- Kaira, K.; Sunose, Y.; Arakawa, K.; Ogawa, T.; Sunaga, N.; Shimizu, K.; Tominaga, H.; Oriuchi, N.; Itoh, H.; Nagamori, S.; et al. Prognostic significance of L-type amino-acid transporter 1 expression in surgically resected pancreatic cancer. Br. J. Cancer 2012, 107, 632–638.
- Okanishi, H.; Ohgaki, R.; Okuda, S.; Endou, H.; Kanai, Y. Proteomics and phosphoproteomics reveal key regulators associated with cytostatic effect of amino acid transporter LAT1 inhibitor. Cancer Sci. 2021, 112, 871–883.
- Okano, N.; Hana, K.; Naruge, D.; Kawai, K.; Kobayashi, T.; Nagashima, F.; Endou, H.; Furuse, J. Biomarker Analyses in Patients with Advanced Solid Tumors Treated with the LAT1 Inhibitor JPH203. In Vivo 2020, 34, 2595–2606.
- Yothaisong, S.; Namwat, N.; Yongvanit, P.; Khuntikeo, N.; Puapairoj, A.; Jutabha, P.; Anzai, N.; Tassaneeyakul, W.; Tangsucharit, P.; Loilome, W. Increase in L-type amino acid transporter 1 expression during cholangiocarcinogenesis caused by liver fluke infection and its prognostic significance. Parasitol. Int. 2017, 66, 471–478.
- Kaira, K.; Sunose, Y.; Oriuchi, N.; Kanai, Y.; Takeyoshi, I. CD98 is a promising prognostic biomarker in biliary tract cancer. Hepatobiliary Pancreat. Dis. Int. 2014, 13, 654–657.
- Yanagisawa, N.; Hana, K.; Nakada, N.; Ichinoe, M.; Koizumi, W.; Endou, H.; Okayasu, I.; Murakumo, Y. High expression of L-type amino acid transporter 1 as a prognostic marker in bile duct adenocarcinomas. Cancer Med. 2014, 3, 1246–1255.
- Janpipatkul, K.; Suksen, K.; Borwornpinyo, S.; Jearawiriyapaisarn, N.; Hongeng, S.; Piyachaturawat, P.; Chairoungdua, A. Downregulation of LAT1 expression suppresses cholangiocarcinoma cell invasion and migration. Cell. Signal. 2014, 26, 1668–1679.
- Kaira, K.; Sunose, Y.; Ohshima, Y.; Ishioka, N.S.; Arakawa, K.; Ogawa, T.; Sunaga, N.; Shimizu, K.; Tominaga, H.; Oriuchi, N.; et al. Clinical significance of L-type amino acid transporter 1 expression as a prognostic marker and potential of new targeting therapy in biliary tract cancer. BMC Cancer 2013, 13, 482.
- Sato, K.; Miyamoto, M.; Takano, M.; Furuya, K.; Tsuda, H. Significant relationship between the LAT1 expression pattern and chemoresistance in ovarian clear cell carcinoma. Virchows Arch. Int. J. Pathol. 2019, 474, 701–710.
- Kaira, K.; Nakamura, K.; Hirakawa, T.; Imai, H.; Tominaga, H.; Oriuchi, N.; Nagamori, S.; Kanai, Y.; Tsukamoto, N.; Oyama, T.; et al. Prognostic significance of L-type amino acid transporter 1 (LAT1) expression in patients with ovarian tumors. Am. J. Transl. Res. 2015, 7, 1161–1171.
- Fan, X.; Ross, D.D.; Arakawa, H.; Ganapathy, V.; Tamai, I.; Nakanishi, T. Impact of system L amino acid transporter 1 (LAT1) on proliferation of human ovarian cancer cells: A possible target for combination therapy with anti-proliferative aminopeptidase inhibitors. Biochem. Pharmacol. 2010, 80, 811–818.
- Kaji, M.; Kabir-Salmani, M.; Anzai, N.; Jin, C.J.; Akimoto, Y.; Horita, A.; Sakamoto, A.; Kanai, Y.; Sakurai, H.; Iwashita, M. Properties of L-type amino acid transporter 1 in epidermal ovarian cancer. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2010, 20, 329–336.
- Thompson, C.; Rahman, M.M.; Singh, S.; Arthur, S.; Sierra-Bakhshi, C.; Russell, R.; Denning, K.; Sundaram, U.; Salisbury, T. The Adipose Tissue-Derived Secretome (ADS) in Obesity Uniquely Induces L-Type Amino Acid Transporter 1 (LAT1) and mTOR Signaling in Estrogen-Receptor-Positive Breast Cancer Cells. Int. J. Mol. Sci. 2021, 22, 6706.
- Ichinoe, M.; Mikami, T.; Yanagisawa, N.; Yoshida, T.; Hana, K.; Endou, H.; Okayasu, I.; Sengoku, N.; Ogata, H.; Saegusa, M.; et al. Prognostic values of L-type amino acid transporter 1 and CD98hc expression in breast cancer. J. Clin. Pathol. 2020, 74, 589–595.
- Bodoor, K.; Almomani, R.; Alqudah, M.; Haddad, Y.; Samouri, W. LAT1 (SLC7A5) Overexpression in Negative Her2 Group of Breast Cancer: A Potential Therapy Target. Asian Pac. J. Cancer Prev. APJCP 2020, 21, 1453–1458.
- Pocasap, P.; Weerapreeyakul, N.; Timonen, J.; Järvinen, J.; Leppänen, J.; Kärkkäinen, J.; Rautio, J. Tyrosine-Chlorambucil Conjugates Facilitate Cellular Uptake through L-Type Amino Acid Transporter 1 (LAT1) in Human Breast Cancer Cell Line MCF-7. Int. J. Mol. Sci. 2020, 21, 2132.
- Shennan, D.B.; Thomson, J. Inhibition of system L (LAT1/CD98hc) reduces the growth of cultured human breast cancer cells. Oncol. Rep. 2008, 20, 885–889.
- Dvorak, V.; Wiedmer, T.; Ingles-Prieto, A.; Altermatt, P.; Batoulis, H.; Barenz, F.; Bender, E.; Digles, D.; Durrenberger, F.; Heitman, L.H.; et al. An Overview of Cell-Based Assay Platforms for the Solute Carrier Family of Transporters. Front. Pharm. 2021, 12, 722889.
- Chien, H.C.; Colas, C.; Finke, K.; Springer, S.; Stoner, L.; Zur, A.A.; Venteicher, B.; Campbell, J.; Hall, C.; Flint, A.; et al. Reevaluating the Substrate Specificity of the L-Type Amino Acid Transporter (LAT1). J. Med. Chem. 2018, 61, 7358–7373.
- Baniasadi, S.; Chairoungdua, A.; Iribe, Y.; Kanai, Y.; Endou, H.; Aisaki, K.; Igarashi, K.; Kanno, J. Gene expression profiles in T24 human bladder carcinoma cells by inhibiting an L-type amino acid transporter, LAT1. Arch. Pharmacal. Res. 2007, 30, 444–452.
- Kume, E.; Mutou, T.; Kansaku, N.; Takahashi, H.; Wempe, M.F.; Ikegami, M.; Kanai, Y.; Endou, H.; Wakui, S. Ultrastructural immunohistochemical study of L-type amino acid transporter 1-4F2 heavy chain in tumor microvasculatures of N-butyl-N-(4-hydroxybutyl) nitrosamine (BBN) induced rat bladder carcinoma. Microscopy 2017, 66, 198–203.
- Oda, K.; Hosoda, N.; Endo, H.; Saito, K.; Tsujihara, K.; Yamamura, M.; Sakata, T.; Anzai, N.; Wempe, M.F.; Kanai, Y.; et al. L-type amino acid transporter 1 inhibitors inhibit tumor cell growth. Cancer Sci. 2010, 101, 173–179.
- Rosilio, C.; Nebout, M.; Imbert, V.; Griessinger, E.; Neffati, Z.; Benadiba, J.; Hagenbeek, T.; Spits, H.; Reverso, J.; Ambrosetti, D.; et al. L-type amino-acid transporter 1 (LAT1): A therapeutic target supporting growth and survival of T-cell lymphoblastic lymphoma/T-cell acute lymphoblastic leukemia. Leukemia 2015, 29, 1253–1266.
- Okunushi, K.; Furihata, T.; Morio, H.; Muto, Y.; Higuchi, K.; Kaneko, M.; Otsuka, Y.; Ohno, Y.; Watanabe, Y.; Reien, Y.; et al. JPH203, a newly developed anti-cancer drug, shows a preincubation inhibitory effect on L-type amino acid transporter 1 function. J. Pharm. Sci. 2020, 144, 16–22.
- Cormerais, Y.; Pagnuzzi-Boncompagni, M.; Schrotter, S.; Giuliano, S.; Tambutte, E.; Endou, H.; Wempe, M.F.; Pages, G.; Pouyssegur, J.; Picco, V. Inhibition of the amino-acid transporter LAT1 demonstrates anti-neoplastic activity in medulloblastoma. J. Cell Mol. Med. 2019, 23, 2711–2718.
- Choi, D.W.; Kim, D.K.; Kanai, Y.; Wempe, M.F.; Endou, H.; Kim, J.K. JPH203, a selective L-type amino acid transporter 1 inhibitor, induces mitochondria-dependent apoptosis in Saos2 human osteosarcoma cells. Korean J. Physiol. Pharm. 2017, 21, 599–607.
- Hafliger, P.; Graff, J.; Rubin, M.; Stooss, A.; Dettmer, M.S.; Altmann, K.H.; Gertsch, J.; Charles, R.P. The LAT1 inhibitor JPH203 reduces growth of thyroid carcinoma in a fully immunocompetent mouse model. J. Exp. Clin. Cancer Res. 2018, 37, 234.
- Enomoto, K.; Sato, F.; Tamagawa, S.; Gunduz, M.; Onoda, N.; Uchino, S.; Muragaki, Y.; Hotomi, M. A novel therapeutic approach for anaplastic thyroid cancer through inhibition of LAT1. Sci. Rep. 2019, 9, 14616.
- Shindo, H.; Harada-Shoji, N.; Ebata, A.; Sato, M.; Soga, T.; Miyashita, M.; Tada, H.; Kawai, M.; Kosaka, S.; Onuki, K.; et al. Targeting Amino Acid Metabolic Reprogramming via L-Type Amino Acid Transporter 1 (LAT1) for Endocrine-Resistant Breast Cancer. Cancers 2021, 13, 4375.
- Satou, M.; Wang, J.; Nakano-Tateno, T.; Teramachi, M.; Suzuki, T.; Hayashi, K.; Lamothe, S.; Hao, Y.; Kurata, H.; Sugimoto, H.; et al. L-type amino acid transporter 1, LAT1, in growth hormone-producing pituitary tumor cells. Mol. Cell Endocrinol. 2020, 515, 110868.
- Ueno, S.; Kimura, T.; Yamaga, T.; Kawada, A.; Ochiai, T.; Endou, H.; Sakurai, H. Metformin enhances anti-tumor effect of L-type amino acid transporter 1 (LAT1) inhibitor. J. Pharm. Sci. 2016, 131, 110–117.
- Maimaiti, M.; Sakamoto, S.; Yamada, Y.; Sugiura, M.; Rii, J.; Takeuchi, N.; Imamura, Y.; Furihata, T.; Ando, K.; Higuchi, K.; et al. Expression of L-type amino acid transporter 1 as a molecular target for prognostic and therapeutic indicators in bladder carcinoma. Sci. Rep. 2020, 10, 1292.
- Higuchi, K.; Sakamoto, S.; Ando, K.; Maimaiti, M.; Takeshita, N.; Okunushi, K.; Reien, Y.; Imamura, Y.; Sazuka, T.; Nakamura, K.; et al. Characterization of the expression of LAT1 as a prognostic indicator and a therapeutic target in renal cell carcinoma. Sci. Rep. 2019, 9, 16776.
- Otsuki, H.; Kimura, T.; Yamaga, T.; Kosaka, T.; Suehiro, J.I.; Sakurai, H. Prostate Cancer Cells in Different Androgen Receptor Status Employ Different Leucine Transporters. Prostate 2017, 77, 222–233.
- Okano, N.; Naruge, D.; Kawai, K.; Kobayashi, T.; Nagashima, F.; Endou, H.; Furuse, J. First-in-human phase I study of JPH203, an L-type amino acid transporter 1 inhibitor, in patients with advanced solid tumors. Investig. New Drugs 2020, 38, 1495–1506.
- Yan, R.; Li, Y.; Muller, J.; Zhang, Y.; Singer, S.; Xia, L.; Zhong, X.; Gertsch, J.; Altmann, K.H.; Zhou, Q. Mechanism of substrate transport and inhibition of the human LAT1-4F2hc amino acid transporter. Cell Discov. 2021, 7, 16.
- Wempe, M.F.; Rice, P.J.; Lightner, J.W.; Jutabha, P.; Hayashi, M.; Anzai, N.; Wakui, S.; Kusuhara, H.; Sugiyama, Y.; Endou, H. Metabolism and pharmacokinetic studies of JPH203, an L-amino acid transporter 1 (LAT1) selective compound. Drug Metab. Pharm. 2012, 27, 155–161.
- Quan, L.; Ohgaki, R.; Hara, S.; Okuda, S.; Wei, L.; Okanishi, H.; Nagamori, S.; Endou, H.; Kanai, Y. Amino acid transporter LAT1 in tumor-associated vascular endothelium promotes angiogenesis by regulating cell proliferation and VEGF-A-dependent mTORC1 activation. J. Exp. Clin. Cancer Res. 2020, 39, 266.
- Kaira, K.; Oriuchi, N.; Imai, H.; Shimizu, K.; Yanagitani, N.; Sunaga, N.; Hisada, T.; Ishizuka, T.; Kanai, Y.; Endou, H.; et al. Prognostic significance of L-type amino acid transporter 1 (LAT1) and 4F2 heavy chain (CD98) expression in early stage squamous cell carcinoma of the lung. Cancer Sci. 2009, 100, 248–254.
- Kaira, K.; Oriuchi, N.; Shimizu, K.; Ishikita, T.; Higuchi, T.; Imai, H.; Yanagitani, N.; Sunaga, N.; Hisada, T.; Ishizuka, T.; et al. Correlation of angiogenesis with 18F-FMT and 18F-FDG uptake in non-small cell lung cancer. Cancer Sci. 2009, 100, 753–758.
- Okubo, S.; Zhen, H.N.; Kawai, N.; Nishiyama, Y.; Haba, R.; Tamiya, T. Correlation of L-methyl-11C-methionine (MET) uptake with L-type amino acid transporter 1 in human gliomas. J. Neurooncol. 2010, 99, 217–225.
- Haining, Z.; Kawai, N.; Miyake, K.; Okada, M.; Okubo, S.; Zhang, X.; Fei, Z.; Tamiya, T. Relation of LAT1/4F2hc expression with pathological grade, proliferation and angiogenesis in human gliomas. BMC Clin. Pathol. 2012, 12, 4.
- Solimando, A.G.; Summa, S.; Vacca, A.; Ribatti, D. Cancer-Associated Angiogenesis: The Endothelial Cell as a Checkpoint for Immunological Patrolling. Cancers 2020, 12, 3380.
This entry is offline, you can click here to edit this entry!
