Cytokines and their receptors have a vital function in regulating various processes such as immune function, inflammation, haematopoiesis, cell growth and differentiation. The interaction between a cytokine and its specific receptor triggers intracellular signalling cascades that lead to altered gene expression in the target cell and consequent changes in its proliferation, differentiation, or activation.
1. Introduction
1.1. Cytokines and Specific Receptors
Cytokines are a broad category of small secretory proteins (<40 kDa) produced by a wide range of cells in response to different stimuli and released for cell signalling. They play vital regulatory roles in diverse immune function, inflammation, haematopoiesis, cell growth and differentiation. Each cytokine has a matching cell-surface receptor. Their interaction in the extracellular environment elicits intracellular signalling cascades leading to altered gene expression in the target cell and consequent biological effects, such as differentiation, proliferation, and activation. Classification is currently based on the type of receptor they bind to. Cytokine receptors are classified into five prominent families: type I cytokine receptors, type II cytokine receptors, immunoglobulin superfamily receptors, tumour necrosis factor (TNF)-like receptors, and chemokine receptors.
1.2. Type I Cytokine Receptors
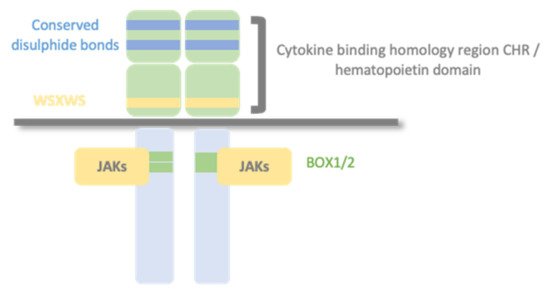
Type I cytokine receptors recognize and respond to type I cytokines and consist of multiple (usually two) transmembrane protein chain modules, similar in their basic structure. Each chain possesses an extracellular domain, also called the hemopoietin domain, involved in ligand/cytokine interaction and a cytoplasmic domain involved in signal transduction. (Figure 1).
Figure 1. Type I cytokine receptors. Type I cytokine receptors consist of multiple (usually two) transmembrane protein chains modules, similar in their basic structure. Each chain possesses an extracellular cytokine binding homology region (CHR), also called the hemopoietin domain, involved in cytokine interaction and a cytoplasmic domain involved in signal transduction. Structurally, the hematopoietin domain comprises fibronectin type III repeats with two pairs of disulfide-linked cysteines and a highly conserved WSXWS motif (X indicates any amino acid). The cytoplasmic domain contains proline-rich Box1/Box2 motifs, which mediate interaction and activation of Janus tyrosine kinases (JAKs).
The primary mechanism for receptor activation is that the binding of the cytokine to the CHR domains leads to dimerization of the receptor ectodomains. Some type I cytokine receptor family members are homodimers when bound to their activating ligand. In contrast, other receptor members are heterodimers, with two or three protein chains, one of them shared by different cytokine receptor complexes. There are three typical class I cytokine receptor protein chains: Glycoprotein 130 (GP130), common beta chain (βc), and common gamma chain (γc).
Aside from membrane-bound cytokine receptors, soluble variants of several cytokine receptors are generated by different molecular mechanisms. A membrane-anchored cytokine receptor can be cleaved by a protease, resulting in a soluble receptor; a differential mRNA splicing can lead to a transcript encoding for a soluble form of the cytokine receptor; a soluble cytokine receptor can be released from cells via exosomes. These soluble receptors modulate the function of cytokines in several ways. They can act as agonistic and antagonistic decoy receptors, forming active receptor/cytokine complexes that can activate cells or compete with their membrane-bound counterparts for the ligand.
1.3. Glycoprotein 130 Cytokine Receptor Family and IL-6 Cytokines
Glycoprotein 130 (GP130) is one of the shared type I cytokine receptor chains and the standard signal-transducing molecule for almost all cytokines of the IL-6 family. The IL-6 family of cytokines comprises 12 members, namely IL-6, IL-11, IL-27, Oncostatin M (OSM), leukaemia inhibitory factor (LIF), ciliary neurotrophic factor (CNTF), cardiotrophin-1 (CT-1), cardiotrophin-like cytokine factor 1 (CLCF1), neuropoietin, IL-31, IL-35 and IL-39.
1.4. The JAK/STAT Pathway
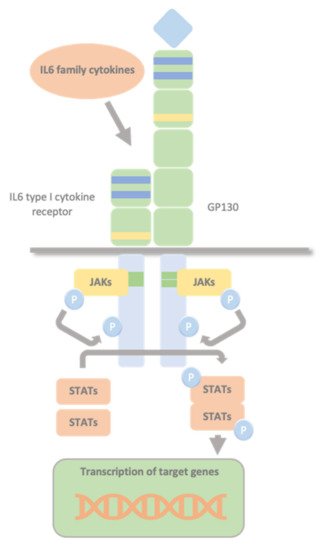
Type I cytokine receptors lack intracellular kinase activity and rely on associated JAKs to transmit intracellular signalling. The binding of the cytokine to its cytokine receptor drives receptor dimerization, oligomerization, and conformational changes that facilitate the activation of the JAKs, constitutively bound to these receptors’ intracellular domains. The intrinsic Box1/2 motifs mediate the interaction and activation of JAK kinase. The activation consists of JAK auto- or transphosphorylation, which subsequently phosphorylate specific tyrosine residues in the intracellular domains of the receptor and subsequent phosphorylation of the receptor by JAKs. This modification creates docking sites for STAT molecules which, once bound to the receptor, are also phosphorylated by JAKs. Phosphorylated STATs dissociate from the receptor, dimerize, and translocate to the nucleus. They control transcription by directly binding to the DNA to mediate transcriptional programs determining fundamental phenotypic changes in the cell (Figure 2).
Figure 2. Glycoprotein 130 Cytokine Receptor Family and IL-6 Cytokines. IL-6 family cytokines use GP130 to transduce their signals through GP130 homodimers or GP130-containing heterodimers with the type I cytokine non-signalling α-receptors (IL-6Rα, IL-11Rα, and CNTFRα), and the signal-transducing receptors (LIFRβ and OSMR). The intrinsic Box1/2 motifs mediate the interaction and activation of JAK kinase. The activation consists of JAK auto- or transphosphorylation, that subsequently phosphorylates specific tyrosine residues in the intracellular domains of the receptor and subsequent phosphorylation of the receptor by JAKs. This creates docking sites for STAT molecules which, once bound to the receptor are also phosphorylated by JAKs. Phosphorylated STATs dissociate from the receptor, dimerize, and translocate to the nucleus, where they control transcription by directly binding to the DNA to mediate transcriptional program determining fundamental phenotypic changes in the cell.
2. Overview of Cardiotrophin-like Cytokine Factor 1/Cytokine Receptor-like Factor-1
2.1. CLCF1 Key Concepts
Cardiotrophin-like cytokine factor 1 (CLCF1) is a member of the IL-6 family and was firstly identified in 1999. CLCF1 consists of three exons and maps to 11q13.2. Its cDNA (RefSeq NM_013246) encodes for a 225 aa protein (RefSeq NP_037378) with a conventional signal peptide spanning from amino acid 1 to 27. The mature form consists of a 198 aa peptide of 22 kDa. Human and murine CLCF1 share a 96% amino acid identity. In addition to features typical of IL-6 family cytokines, including neurotropic effects, it shows B cell-stimulating capability. Therefore, it was named novel neurotrophin-1yB cell-stimulating factor-3 or cardiotrophin-like cytokine (CLC), according to its high similarity with cardiotrophin-1 (CT-1), another member of the IL-6 family. It is expressed mainly in lymphoid tissue, such as lymph nodes and spleen, in humans and mice, indicating relevance to the immune system. However, it is also present in various organs, like most IL-6 family members. This finding may indicate a functional pleiotropy, a characteristic of cytokines in general.
2.2. CRLF1 Key Concepts
The human CRLF1 gene consists of nine exons and maps to 19p13.11. Its cDNA (RefSeq NM_004750) encodes a 422 aa precursor protein of 46 kDa (RefSeq NP_004741) with a 37 amino acid putative signal peptide. Human and murine CRLF1 share a 96% amino acid identity. It belongs to the cytokine type I receptor family, containing a hematopoietin domain with a pair of fibronectins (FBN) type III modules and a highly conserved WSXWS motif. This extremely high level of conservation among vertebrates during recent evolution, as also present in CLCF1, indicates an important functional role for both. The expression pattern of human and mouse CRLF1 suggests a role for the protein in the immune system and foetal development. Human CRLF1 mRNA is predominantly expressed in the adult spleen, thymus, lymph node, appendix, bone marrow, stomach, placenta, heart, thyroid, ovary, fibroblasts, and foetal lung. It is up-regulated by pro-inflammatory cytokines such as TNF-a, IL-6, and IFN-g, suggesting that hCRLF1 may be involved in regulating the immune system during an inflammatory response. Whole-mount in situ hybridization in mice embryos at different time points in the developing embryo revealed Crlf1 expression at multiple sites starting from E9.5.
2.3. CRLF1/CLCF1/CNTFRα Complex
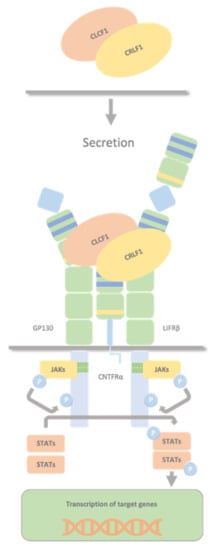
CLCF1 and CRLF1 forms a secreted heterodimer CRLF1/CLCF1 that activates the CNTF receptor (a tripartite receptor complex formed by CNTFRα, GP130 and LIFRβ), with the induction of downstream signalling events, including activation of the JAK1/STAT3 pathway (Figure 3).
Figure 3. CRLF1/CLCF1/CNTFRα complex. The secreted heterodimer CRLF1/CLCF1 activates the CNTF receptor (a tripartite receptor complex formed by GP130, CNTFRα, and LIFRβ), with the induction of downstream signalling events, including activation of the JAK1/STAT3 pathway. Both CLCF1 and CRLF1 have a binding site for CNTFRα and interact with each other.
2.4. CRLF1/CLCF1/CNTFRα/SorLA Complex
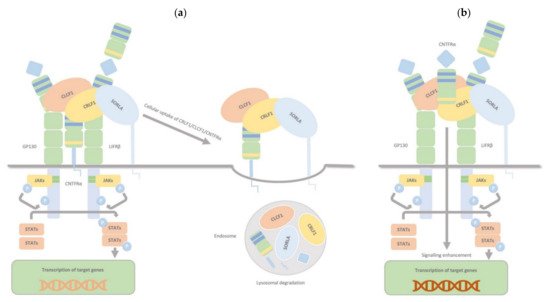
CRLF1, aside from the two binding sites for CLCF1 and CNTFRα, has another one for sortilin-related receptor 1 (SorLA), and it can bind all targets simultaneously. The CRLF1/CLCF1 and CRLF1/CLCF1/sCNTFRα complexes bind to SorLA via the CRLF1 subunit. Depending on the binding partner, this binding can either modulate the cellular response to CRLF1/CLCF1 by mediating CRLF1-dependent endocytosis and subsequent lysosomal degradation (membranous CNTFRα) or enhance signalling, particularly in CNTFR-deficient cells, by binding and accumulating complexes of CRLF1/CLCF1/ sCNTFRα at the cell membrane (soluble form of CNTFRα) (Figure 4). Thus, through interaction with CRLF1, SorLA regulates CLCF1/CNTFRα signalling and turnover.
Figure 4. CRLF1/CLCF1/CNTFRα/SorLA complex. CRLF1 has at least three independent binding sites: one for CLCF1, one for CNTFRα, and one for SorLA, and it can bind all its targets simultaneously. The complexes CRLF1/CLCF1 and CRLF1/CLCF1/sCNTFRα bind to SorLA via the CRLF1 subunit, and this binding, according to its partners, can either (a) (membrane-bound subunit CNTFRα) modulate the cellular response to CRLF1/CLCF1 by mediating CRLF1-dependent endocytosis and subsequent lysosomal degradation or (b) (soluble form of CNTFRα) enhance signalling, notably in CNTFR-deficient cells, by binding and concentrating complexes of CRLF1/CLCF1/ sCNTFRα on the cell membrane. Therefore, through interaction with CRLF1, SorLA regulates CLCF1/CNTFRα signalling and turnover.
2.5. CRLF1/p28 Complex
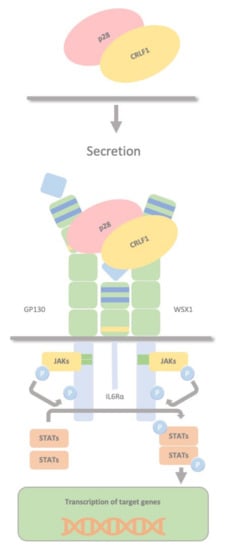
CRLF1 can also chaperone the secretion of p28 to create a composite cytokine that binds and signals through a different complex, the IL27R, formed by GP130, the WSX1 receptor and the interleukin 6 receptor (IL-6R). The IL-27 complex derives from the association of the cytokine p28 with the soluble cytokine receptor EBV-induced gene 3 (EBI3). This complex activates STAT1 and STAT3 phosphorylation and possibly SHP2/Ras/MAPK signalling within the target cell (Figure 5). Dendritic cells produce this complex, which stimulates NK cells, and inhibits CD4 T cell proliferation, highlighting the role of dendritic cells in regulating NK and T cell functions. P28 does not require CRLF1 to initiate signalling through this receptor complex; therefore, CRLF1 acts only as a chaperone in this case. Furthermore, p28/CRLF1 is active on mouse B cells and induces plasma cell differentiation.
Figure 5. CRLF1/p28 complex. The IL-27 complex is formed by the association of the cytokine p28 with the soluble cytokine receptor EBV-induced gene 3 (EBI3). CRLF1 can chaperone the secretion of p28 to form a third composite cytokine that binds and signals through a different complex containing GP130, the WSX1 receptor and the interleukin 6 receptor (IL-6R). This also activates STAT1 and STAT3 phosphorylation and possibly SHP2/Ras/MAPK signalling within the target cell. This complex is produced by dendritic cells, stimulates NK cells, and inhibits CD4 T cell proliferation, highlighting a role in regulating NK and T cell functions by dendritic cells.
3. CRLF1 and CLCF1 in Development, Health and Disease
3.1. CS/CISS1 and CS/CISS2
Mutations in CRLF1 or CLCF1 are associated with Crisponi/cold-induced sweating syndrome (CS/CISS). More than 40 disease-causing mutations in CRLF1 (CS/CISS1, MIM# 601378) have been identified in 96 CS/CISS1 individuals. In contrast, only three cases have been described with pathogenic mutations in CLCF1 associated with CS/CISS2 (MIM# 610313). CS/CISS’s main neonatal clinical features include febrile crises with temperatures up to 42 °C (hyperthermia), orofacial/laryngeal muscle constrictions, including paroxysmal contractions in the facial and oropharyngeal muscles, dysphagia, and camptodactyly. In adolescence, hyperthermia spontaneously improves; cold-induced sweating develops at ambient temperatures below 20 °C.
4. Conclusions and Outlook
Considering the pivotal role that cytokines and their receptors, either transmembrane or soluble, play in health and disease, they are all promising candidates for therapeutic options. Since the identification of CRLF1 and CLCF1 and their involvement in CS/CISS, there has been a growing understanding of their pleiotropic roles in development, health and other diseases. There is still much to learn about their role in biological function and the potential therapeutic applications of these cytokines. However, most of this knowledge comes from sparse studies, mostly microarray and transcriptional studies, in various pathological conditions. Further studies are needed to unravel their specific functions and underlying physiopathological mechanisms and signalling pathways to develop knowledge-based therapies that could help treat the rare and more common diseases they are involved in.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23020992