Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Dermatology
The underlying basis of psoriasis is skin inflammation (and, in the case of psoriatic arthritis, inflammation of the connective tissue which makes up the joints and the joint ligaments). The characteristic features of skin inflammation include hyperplasia of the epidermis, parakeratosis, and an inflammatory infiltration consisting of dendritic cells, macrophages, T-lymphocytes, and neutrophils [9]. Abnormalities in the skin’s immune response are responsible for the development of the inflammation.
- psoriasis
- biological treatment
1. Etiopathogenesis of Psoriasis
The underlying basis of psoriasis is skin inflammation (and, in the case of psoriatic arthritis, inflammation of the connective tissue which makes up the joints and the joint ligaments). The characteristic features of skin inflammation include hyperplasia of the epidermis, parakeratosis, and an inflammatory infiltration consisting of dendritic cells, macrophages, T-lymphocytes, and neutrophils [9]. Abnormalities in the skin’s immune response are responsible for the development of the inflammation. Endogenous risk signals cause the expression of the proinflammatory cytokines responsible for maintaining inflammation in the area of the skin. Figure 1 shows the differences between normal and lesional skin. Characteristic histological changes for cutaneous psoriasis can be observed. In diseased skin, psoriatic plaques have a compact and strongly thickened stratum corneum layer of the epidermis, no granular layer, an enlarged spinous layer, and an accumulation of neutrophils in the stratum corneum and the spinous layer of the epidermis. Within skin with psoriasis lesions, there is an accumulation of dendritic cells and macrophages, which are the source of inflammatory cytokines, which also contribute to the differentiation of naive T lymphocytes into the Th1 or Th17 subpopulations.
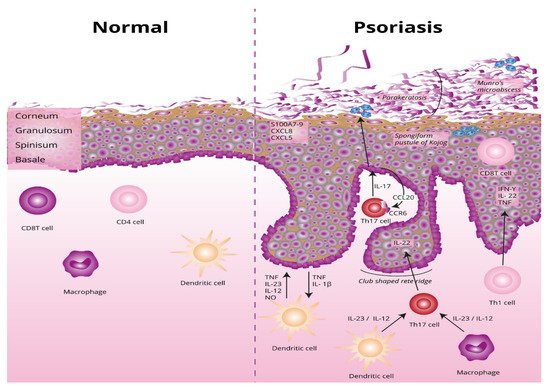
Figure 1. Differences between normal and lesional skin. TNF, tumor necrosis factor alpha; IL-23, interleukin 23; IL-12, interleukin 12; NO, nitric oxide; IL-1β, interleukin 1 beta; Th17, T helper 17; Th, T helper; CCL20, C-C motif chemokine ligand 20; CCR6, C-C motif chemokine receptor 6; CXCL5, C-X-C motif chemokine ligand 5; CXCL8, C-X-C motif chemokine ligand 8; S100A7-, psoriasin.
1.1. Parakeratosis
During the course of psoriasis, the principal symptoms are skin lesions that are reddish and brown-reddish flat protruding lumps of different sizes, covered with silvery-grey scales. They are created as a result of parakeratosis, which is a kind of keratinization characterized by preservation of the cell nucleus by the cells that make up the stratum corneum layer of the epidermis [10]. This inflammatory process [11] leads to replacing annular squames with nucleated cells.
1.2. Factors Precipitating the Development of Psoriasis and Stimulating the Development of Changes
The etiopathogenesis of psoriasis involves genetic factors (numerous genes significantly increasing the risk of contracting psoriasis have been described), environmental factors (such as stress, smoking, injuries, drugs, or bacterial flora), and immunological factors (an abnormal immune response from the T-lymphocytes, dendritic cells, and/or keratinocytes). Factors that evoke the development of changes are slight skin injuries, smoking, alcohol consumption, stress, conditions that cause severe hormonal changes (such as menstruation or menopause in women), some drugs, and infections [12].
1.2.1. Genetic Factors
Psoriasis is certainly polygenic. Advancements in science have made significant progress in understanding the genetics of this type of dermatosis [12]. Nine different regions containing genes predisposing a person to the induction and development of the disease in question have been discovered, known as PSORS1–9, of which the most important role is played by the PSORS1 region. It is located on chromosome 6p21, where genes belonging to the major histocompatibility complex (MHC) are located, of which three (HLA-C, CCHCR1, and CDSN) are highly polymorphic. Numerous studies have confirmed their association with the development of psoriasis [12,13,14]. For example, Caputo et al. [14] confirmed that psoriasis is a very complex disease in which genetic, epigenetic and molecular factors play a key role. An important issue in the etiopathogenesis of the disease and the design of new drugs is to consider the fact of the mutual overlapping of many signaling pathways. Therefore, further research on psoriasis should consider the integration of large-scale epigenomic data and the three-dimensional organization of the genome into the so-called systems biology. This is possible thanks to the advancement of the omics era [14].
1.2.2. Environmental Factors
The important role of environmental factors in the development of lesions underlines the complex nature of psoriasis. They play a key role in the initiation of the disease and its progress [15]. The environmental stressors that play a key role in the induction and development of psoriasis include noise, air pollution, improper diet, alcohol abuse, smoking, and drug addiction. Physiological stressors that contribute to the development of psoriasis are infections, primarily bacterial, and injuries, e.g., mechanical damage to the epidermis (the so-called Köbner effect) [16]. In turn, the psychological stressors include depression, incorrect social relations, family conflict, and professional problems. However, knowledge on this subject remains fragmented [17].
1.2.3. Stress
In a study involving patients diagnosed with psoriasis, as many as 60% were deeply convinced that stress was the causative factor of their disease [18]. In other studies, it has been reported that psychological stress precedes the onset of the disease in 44% of psoriasis patients and initiates recurrent exacerbations in up to 88% of patients [19]. However, in some cases, no clear relationship between stress and disease exacerbations was found [20]. A marked increase in the severity of psoriasis is most often observed about 1 month after exposure to the stressors [21]. The vast majority of patients who report stress-induced psoriasis cite the feeling of cosmetic disfigurement and social stigmatization as the primary cause. The accompanying depressive disorders and reduced quality of life are especially pronounced in women [22].
1.2.4. Pharmacotherapy of Comorbidities
Diseases comorbid with psoriasis cause an increased need for pharmacotherapy in a large group of patients.
Multiple preparations are believed to be possible psoriasis-worsening agents. The disease may appear de novo or be exacerbated [23].
Unfortunately, in clinical practice, it is usually difficult to link psoriasis outbreaks with medications. This is because the latency between treatment initiation and the onset of psoriatic skin lesions is different for many medications used for comorbid conditions. Moreover, with age, the phenomenon of polypragmasy increases significantly [23,24].
The most common pharmacological causes of psoriasis include: beta blockers, lithium salts, antismall drugs, angiotensin-converting enzyme inhibitors, and nonsteroidal anti-inflammatory drugs.
1.2.5. Infections
Exacerbation of the course of psoriasis after an infection is associated with the so-called superantigens, which include bacterial, viral, and parasitic antigens. The similarity between the antigens of microorganisms and epidermal autoantigens may play an important role [25]. Among the infectious factors that can stimulate the formation of psoriasis lesions, the most important role is assigned to the Group A beta-hemolytic streptococci. Streptococcal infections of the upper respiratory tract are most often responsible for the acute appearance of psoriatic lesions in the droplet form of Type I psoriasis. Eruptions often appear quickly, usually within 2–4 weeks after bacterial contamination. Although the lesions are usually self-limiting, they may recur with subsequent streptococcal infections. Therefore, tonsillectomy may be a potential therapeutic option in patients with drug-resistant psoriasis accompanied by frequent episodes of tonsillitis [26,27].
1.2.6. Nicotinism and Alcohol Consumption
Psoriasis patients often report a current or previous addiction to nicotine. Smoking may not only aggravate the course of existing psoriasis but may also increase the risk of de novo disease. A cross-sectional study showed that smokers of more than 20 cigarettes a day had twice the risk of developing severe psoriasis with a reduced likelihood of periods of remission than smokers of less than 10 cigarettes a day [28,29].
The relationship between alcohol consumption and psoriasis has long been controversial. Early research negated such connections, but observations carried out at the end of the 20th century showed significant relationships [30]. Alcohol may be a factor both initiating and intensifying inflammation by stimulating the multiplication of lymphocytes and the production of proinflammatory cytokines. In addition, alcohol can directly induce the proliferation of keratinocytes and increase the mRNA expression of genes related to this phenomenon through the production of proteins such as alpha5 integrin, cyclin D1, and the keratinocyte growth factor receptor (KGFR) [31,32,33]. To date, a limited number of studies have looked at the relationship between the severity of psoriasis and alcohol consumption; however, the observations made thus far seem to indicate the existence of such a relationship [30,31,32].
2. Biological Drugs Used in the Treatment of Psoriasis
According to the recommendations of the Polish Dermatological Society, the evaluation of the severity of the disease symptoms is conducted based on the Psoriasis Area and Severity Index (PASI), Body Surface Area (BSA), and the Dermatology Life Quality Index (DLQI) [34]. Topical treatment is the only recommended treatment method for patients with mild psoriasis, who score no more than 10 points on the abovementioned scales. The most used topical treatments are based on vitamin D derivatives and steroids [35]. Patients with diagnosed plaque psoriasis of moderate or acute severity, and those with arthropathic psoriasis who do not react to traditional treatment qualify for biological treatment [34,36]. Although the clinical condition of 70–89% of patients [3] allows for the use of topical treatment, which has less severe side effects, these preparations require time-consuming application [34]. Classical systemic therapies include numerous drugs containing methotrexate, cyclosporine A, and retinoids [36,37]. The current work aimed to focus on the neurological side effects of biological drugs used in the treatment of psoriasis in connection with the increasingly common occurrence of psoriasis within the population [38], as well as the increasing availability of biological treatment. The biological drugs currently used in the treatment of psoriasis can be divided into several groups depending on their mechanism of function (Table 1).
Table 1. Classification of antipsoriatic drugs by their biological influence.
| Group | Marketed Formulations | Biological Influence of the Drug |
|---|---|---|
| TNF-alpha inhibitors |
|
Patients with psoriasis exhibit an excessive production of TNF-alpha in the skin as well as the joints. This is a proinflammatory cytokine that acts through by stimulating the release of numerous proinflammatory factors, which ultimately leads to inflammatory infiltration in the area of the skin. Through the inhibition of this cytokine, the inflammation in the skin area is reduced [39]. |
| IL-12 and IL-23 inhibitors |
|
IL-12 and IL-23 are constructed from a common p40 subunit, which ustekinumab acts against. IL-12 stimulates NK (natural killer) cells and differentiation of CD4+ T cells towards the Th1 phenotype. Ustekinumab, if it is unable to attach to IL-12 or IL-23, which are attached to the IL-12Rβ1 receptors on the cell surface, does not affect complement activity and is not involved in antibody-mediated cytotoxicity of the receptor cells. Ustekinumab can exert its clinical effects in psoriasis and psoriatic arthritis by disrupting the Th1 and Th17 cytokine pathways that play a key role in the pathology of these diseases [40]. |
| IL-23 inhibitors |
|
Recent studies indicated that IL-23 is the most important cytokine in the pathogenesis of psoriasis, as it induces the differentiation of naïve T lymphocytes towards the Th17 phenotype and thus to the formation of psoriatic plaque. The newest p19 inhibitor of IL-23 is risankizumab, which has a good safety profile, less frequent use, and suitable efficacy in severe psoriasis [41]. |
| IL-17 inhibitors |
|
IL-17 is a cytokine that causes an increase in the expression of factors such as TNF-alpha, stimulating the development of inflammatory infiltration. Blocking IL-17 causes a significant reduction in infiltration [40]. |
| T-lymphocyte inhibitors |
|
In the area of skin changes, there are numerous T-lymphocytes with impaired function. This causes the stimulation of an improper inflammatory reaction [42]. The cytotoxic activity of abatacept on T-lymphocytes causes a decrease in their population, resulting in a reduction of the inflammation in the area of psoriatic changes [43]. |
This entry is adapted from the peer-reviewed paper 10.3390/life12010118
This entry is offline, you can click here to edit this entry!
