Energy demand is constantly growing, and, nowadays, fossil fuels still play a dominant role in global energy production, despite their negative effects on air pollution and the emission of greenhouse gases, which are the main contributors to global warming. An alternative clean source of energy is represented by the lignocellulose fraction of plant cell walls, the most abundant carbon source on Earth. To obtain biofuels, lignocellulose must be efficiently converted into fermentable sugars. In this regard, the exploitation of cell wall lytic enzymes (CWLEs) produced by lignocellulolytic fungi and bacteria may be considered as an eco-friendly alternative. These organisms evolved to produce a variety of highly specific CWLEs, even if in low amounts. For an industrial use, both the identification of novel CWLEs and the optimization of sustainable CWLE-expressing biofactories are crucial.
- cell wall lytic enzymes
- lignocellulose
- sustainable biofactory
- heterologous expression
- microalgae
- biofuel
- plant immunity
1. Introduction
The plant cell wall is a complex and heterogeneous structure composed of polysaccharides and phenolic compounds assembled in two distinct layers called primary and secondary cell walls [1]. Clusters of cell walls constitute lignocellulose, a heterogeneous material mainly composed by lignin, hemicellulose, and cellulose; in addition, to provide structural support to the plant, it represents a powerful barrier against both biotic and abiotic stresses [2]. In order to open a breach and depolymerize the cell wall polysaccharides into simple sugars, microbes have evolved specialized enzymes, referred to as cell wall lytic enzymes (CWLEs). The conversion of lignocellulose into fermentable sugars by using CWLEs may be considered as an eco-friendly alternative compared to degradative methods based on chemical treatments. Moreover, the broad substrate specificity of many CWLEs makes these enzymes highly versatile and, thus, exploitable in other important fields such as agriculture, food processing, and medicine. However, the industrial use of CWLEs is characterized by a high cost and low efficiency. Here, recent advances in the heterologous expression of CWLEs as well as some of their possible biotechnological applications will be discussed. In order to support a sustainable biofactory of CWLEs, we will focus on the expression system in plants, since it is characterized by a high productivity/cost ratio and it consumes atmospheric CO2 through photosynthesis, thus positively impacting global warming.
2. Production and bio-application of CWLEs
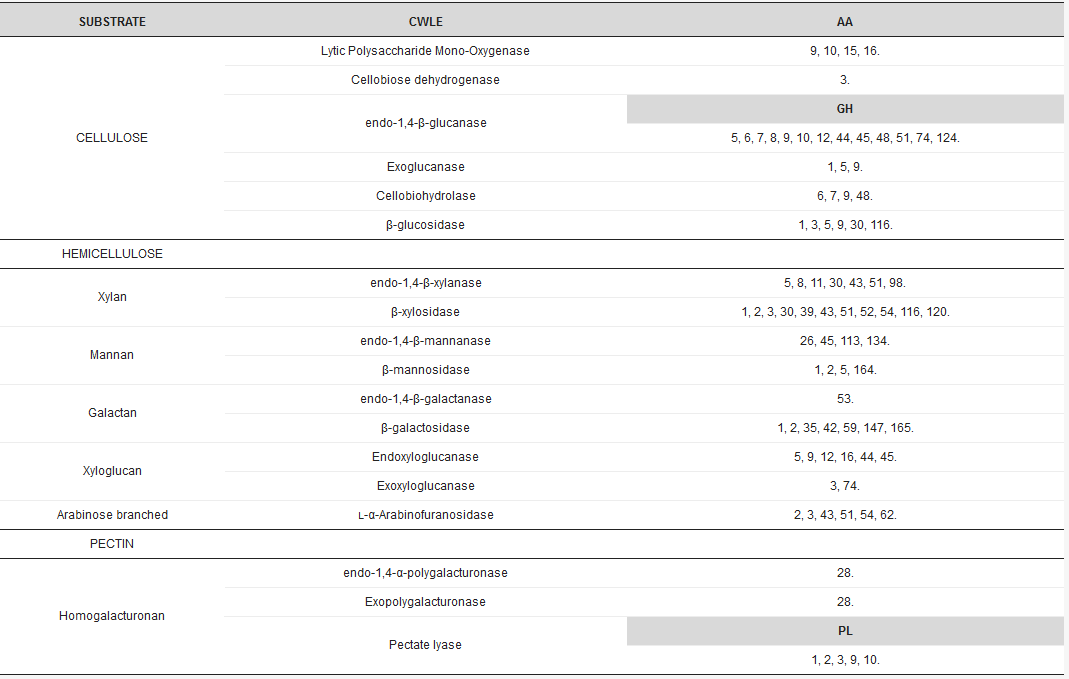
Arrays of CWLEs are secreted by microbes to efficiently hydrolyze the variety of polysaccharides in plant cell walls into simple sugars [3]. Ligninases, hemicellulases, and pectinases, together with cellulolytic enzymes, are required to efficiently hydrolyze the lignocellulosic biomass. CWLEs are classified in the CAZy database (www.cazy.org), where glycoside hydrolases, the most numerous class of carbohydrate active enzymes, are represented by more than 160 different families (Table 1).
Table 1. Distribution of conserved domains in major cell wall lytic enzymes (CWLE) families. Auxiliary activity (AA), polysaccharide lyase (PL), and glycoside-hydrolase (GH) domains are indicated. Numeration of conserved domains is in accordance with the CAZy Database (www.cazy.org).
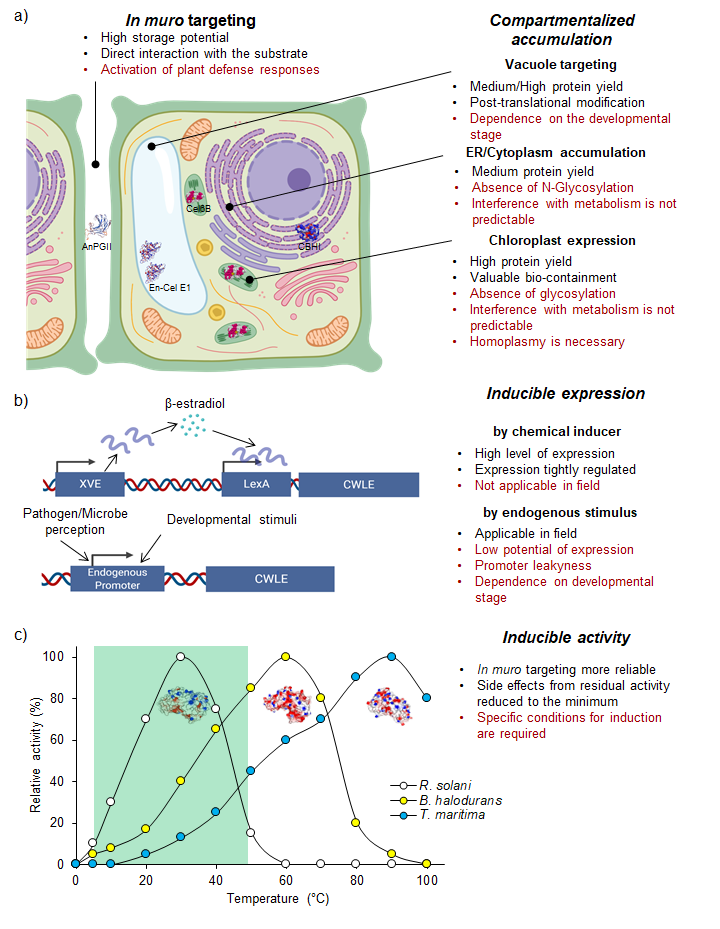
Amongst the various organisms producing CWLEs, lignocellulolytic fungi and bacteria are the most relevant. These organisms are highly specialized in degrading the different components of lignocellulose since they use monosaccharides derived from the plant cell wall to survive and proliferate; thus, they represent a precious source of CWLEs with different substrate specificities and enzymatic characteristics. In general, these organisms evolved to produce a variety of CWLEs in sufficient amounts for their own subsistence. An overall distinction of such organisms could be done based on their lifestyle, for instance, specialized (i) in degrading the cell wall from living plants, as in the case of plant pathogens, or (ii) in degrading dead plant matter, as in the case of saprophytes. However, the exploitation of wild-type microbes as biofactories to obtain CWLEs has a main limitation represented by the low level of enzyme production. This strongly impacts the cost of CWLEs obtained from wild-type hosts, making the entire process of enzymatic degradation less sustainable. Heterologous expression of CWLEs using Escherichia coli, yeasts, and plants may be a valid alternative. To date, different bacteria and yeast species have been used as expression hosts for CWLE production. These microorganisms differ significantly in their cell wall structure and subcellular compartments, thus affecting both protein secretion and expression efficiency. The low levels of soluble proteins and the poor secretion ability represent the main limitations of Gram-negative bacterial hosts. Alternatively, different species belonging to Gram-positive bacteria were selected as expression hosts since they secrete the recombinant proteins more efficiently than Gram-negative bacteria [4][5]. In eukaryotic organisms, yeasts are eligible hosts for overcoming the solubility and secretion limits that affect heterologous expression in the bacterial system. Nowadays, plant expression of microbial CWLEs is a major challenge that biotechnologists are facing. Plants are desirable expression hosts since they are characterized by low production costs and high productivity. Moreover, they consume atmospheric CO2 through photosynthesis, which positively impacts global warming and points to the plant expression system as a valuable green alternative. The in muro targeting of CWLEs may enhance the hydrolysis of cell wall polysaccharides, allowing an efficient conversion of fermentable sugars into biofuel-related compounds [6]. However, expression of CWLEs using plants as a heterologous system may impart unwanted and undesired side effects. CWLEs are produced by microbial pathogens to open a breach in the cell wall, concomitantly supporting the infection process [7]. Therefore, the uncontrolled in planta expression of CWLEs may result in impaired growth, reduced productivity, and lethality [8][9][10]. In order to circumvent these undesired effects, different CWLEs expression strategies may be adopted, such as (i) compartmentalized expression/accumulation, (ii) inducible gene expression, (iii) inducible enzymatic activity, and (iv) use of plant hosts that are not sensitive to CWLE activity (Figure 1).
Figure 1. Plant production of microbial CWLEs. Production of CWLEs as obtained by (a) constitutive expression and compartmentalization or (b) inducible gene expression. Advantages and disadvantages of each strategy are indicated in black and red, respectively. In (a), the represented CWLEs were successfully produced upon compartmentalized expression. (c) Theoretical optimal temperature of XynA from the mesophilic fungus R. solani, from the thermophilic bacterium B. halodurans, and from the hyperthermophilic bacterium T. maritima, here reported as a representative set of CWL isoenzymes. Activity of XynA from T. maritima is strongly reduced in the temperature range of plant growth (green box) and increases the chance of preserving the productivity of the transgenic plant. AnPGII: endo-1,4-α-polyglacturonase II from A. niger; Cel6B: endo-1,4-β-glucanase from T. fusca; En-Cel E1: endo-1,4-β-cellulase from A. cellulolyticus; CBHI: cellobiohydrolase I from T. reseei.
Another attractive strategy resides in the use of microalgae as a platform for the expression of CWLEs. Microalgae are promising expression hosts since they are characterized by a relatively fast growth cycle, and their cultivation is less expensive compared to that of other microorganisms (e.g., bacteria and yeasts) [11]. Moreover, differently from higher plants, microalgae do not require arable lands for their cultivation, thus avoiding the loss of areas that may be employed in the agri-food sector. Contrary to plant cells, some species of unicellular green algae, such as Chlamydomonas reinhardtii, possess a cell wall mainly constituted by proteins [12]; thus, the lack of polysaccharides in their cell wall circumvents the deleterious effects of expressing CWLEs in plants.
The use of plant-expressed CWLEs can be exploited in different industrial and agricultural processes in order to reduce costs and obtain more environmentally and human-safe products. The latest advances in the biotechnological application of CWLEs in the production of second- and third-generation biofuels, medical and nutraceutical fields, food processing, and enhancement of plant resistance to pathogens are shown in Figure 2a. Four major factors contribute to decreasing the enzymatic degradation efficiency of lignocellulose: (i) incomplete knowledge of the reactions and of the enzymes at the basis of such a process, (ii) the hydrophobic nature of lignin and cellulose that hinders the accessibility of glycoside hydrolases to the substrate, (iii) the broad heterogeneity of hemicellulose and pectin that requires many specialized CWLEs for their efficient depolymerization [13], and (iv) the property of lignin and of certain oligosaccharides to act as CWLE inhibitors. Enzymatic hydrolysis of lignocellulosic biomass can be improved by using selected mixtures of CWLEs and optimized reaction conditions. Under this perspective, the choice of proper enzymatic blend is a critical step, since the number of CWLEs is constantly increasing and part of them are characterized by novel and unknown functions. Soluble sugars obtained from agricultural feedstock (e.g., bagasse, straw, corn cob, grass, bran) as well as the same residual biomass (insoluble) upon CWLE treatment can be used as feed for sustaining the fermentative process of yeasts in order to produce ethanol [14]. Enzymatically treated lignocellulose can be used to produce other forms of biofuels, such as those obtained from oleaginous microalgae, namely, third-generation biofuels. Alternatively, agricultural feedstock can be used as feed for promoting the anaerobic digestion of methanogenic bacteria [15](Figure 2b).
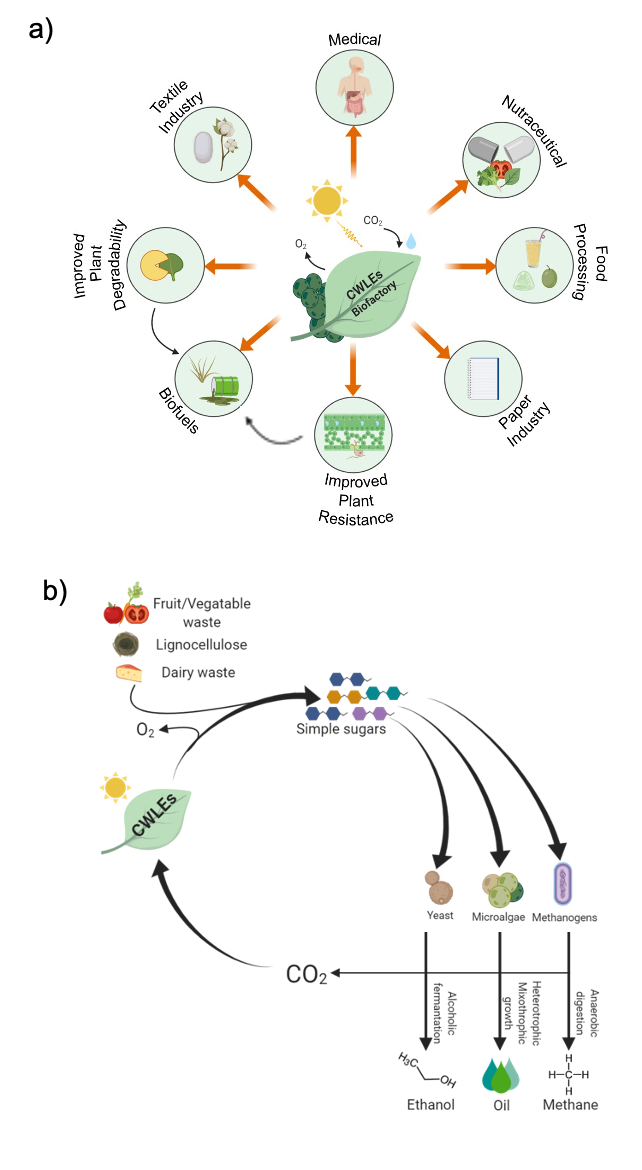
Figure 2. Biotechnological applications of CWLEs. (a) Possible applications of plant-expressed CWLEs in agriculture, industrial, and medical fields; both an increased degradability and biomass productivity positively impact the release of fermentable sugars from plant biomass. (b) Use of plant-expressed CWLEs for the production of second- and third-generation biofuels; sugars released from waste materials upon CWLE-treatment are used as feed for yeasts, microalgae, and methanogenic bacteria. CO2 emitted from these metabolic processes may be converted into CWLEs and O2 by the plant biofactory.
Expression of CWLEs in crop plants and microalgae has two major advantages: the low production costs and their GRAS (Generally Recognized As Safe) designation, which allows exploitation of such CWLEs for the production of nutraceuticals and bio-active compounds for human use. CWLEs are a powerful resource for food biotechnology because of their applicability in a broad range of processes. Fruit and vegetable juice clarification, viscosity reduction of nectars, modification of organoleptic properties, carotenoid extraction, olive oil extraction, and quality improvement of bakery products are the processes in which CWLEs are commonly exploited. Plant expression of CWLEs can trigger the host defense, rendering their endogenous accumulation as self-deleterious for plants. Under this perspective, plant expression of CWLEs resembles a double-edged sword: on one side, activation of defense responses may confer increased resistance against pathogens but, on the other side, may strongly affect plant fitness and productivity, likely because of the well-known growth–defense trade-off [16][17]. A valid strategy to overcome this issue may reside in a balanced activation of defense responses, capable of guaranteeing protection against microbes by concomitantly avoiding exaggerated and deleterious immune reactions. Notably, plant expression of CWLEs capable of triggering defense responses can be used as an eco-friendly alternative to pesticides, preventing the spreading of disease in plants and lowering the costs for managing pests (Figure 2a).
3. Conclusions
An ideal bio-factory of CWLEs should be characterized by a high yield of recombinant protein, sustainable manufacturing, and a reduced production time and cost. In order to select the most appropriate production platform, the type of CWLE to express and its biotechnological purpose represent additional factors to be considered. Although bacteria and yeasts can produce high yields of recombinant proteins in very short time, expensive fermentation procedures are required to obtain high-level expressions, negatively impacting the sustainability of the entire process. Furthermore, CWLEs from lignocellulolytic fungi can be characterized by the presence of disulfide bonds and glycosylations; therefore, the expression in bacteria and yeasts could result in misfolded or hyperglycosylated enzymes, respectively. Importantly, the use of CWLEs from microbial biofactories in food processing, nutraceutical manufacturing, and the medical field implies expensive purification procedures and strict quality controls, since bacterial contaminants, as well as yeast glycosylation, may cause allergic reactions in humans. On the other hand, plant biofactories are both a cheaper and more eco-friendly alternative, and, noteworthy, they can guarantee proper post-translational modifications to the eukaryotic proteins. Moreover, they are GRAS organisms, allowing the obtainment of high-value products at reduced production costs. Although the plant production of CWLEs may affect the fitness of the expression host, mainly because of their intrinsic phytopathogenic nature, such deleterious effects can be avoided by the different expression strategies here reported. However, plants need arable land for their growth and longer times to produce significant amounts of recombinant proteins. Under this perspective, microalgae may represent the best compromise since their cultivation does not subtract arable lands from the agri-food sector, and they are characterized by faster growth rates compared to plants. It is worth noting that genetic manipulation of microalgae has been less investigated; therefore, further optimization is still required for improving the expression stability and protein yield of microalgal-based biofactories. In conclusion, the most appropriate CWLE expression host should be determined on a case-by-case basis, taking into account both the characteristics of the specific CWLE to express and its application field.
This entry is adapted from the peer-reviewed paper 10.3390/app9235012
References
- Keegstra, K. Plant cell walls. Plant Physiol. 2010, 154, 483–486.
- Hamann, T. Plant cell wall integrity maintenance as an essential component of biotic stress response mechanisms. Front. Plant Sci. 2012, 3, 77.
- Horn, S.J.; Vaaje-Kolstad, G.; Westereng, B.; Eijsink, V.G. Novel enzymes for the degradation of cellulose. Biotechnol. Biofuels 2012, 5, 45.
- Morello, E.; Bermúdez-Humarán, L.G.; Llull, D.; Solé, V.; Miraglio, N.; Langella, P.; Poquet, I. Lactobacillus lactis, an efficient cell factory for recombinant protein production and secretion. J. Mol. Microbiol. Biotechnol. 2008, 14, 48–58.
- Pohl, S.; Harwood, C.R. Heterologous Protein Secretion by Bacillus Species. In Advances in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2010; Volume 73, pp. 1–25.
- Li, Q.; Song, J.; Peng, S.; Wang, J.P.; Qu, G.Z.; Sederoff, R.R.; Chiang, V.L. Plant biotechnology for lignocellulosic biofuel production. Plant Biotechnol. J. 2014, 12, 1174–1192.
- Kubicek, C.P.; Starr, T.L.; Glass, N.L. Plant Cell Wall–Degrading Enzymes and Their Secretion in Plant-Pathogenic Fungi. Annu. Rev. Phytopathol. 2014, 52, 427–451.
- Benedetti, M.; Pontiggia, D.; Raggi, S.; Cheng, Z.; Scaloni, F.; Ferrari, S.; Ausubel, F.M.; Cervone, F.; De Lorenzo, G. Plant immunity triggered by engineered in vivo release of oligogalacturonides, damage-associated molecular patterns. Proc. Natl. Acad. Sci. USA 2015, 112, 5533–5538.
- Capodicasa, C.; Vairo, D.; Zabotina, O.; McCartney, L.; Caprari, C.; Mattei, B.; Manfredini, C.; Aracri, B.; Benen, J.; Knox, J.P.; et al. Targeted Modification of Homogalacturonan by Transgenic Expression of a Fungal Polygalacturonase Alters Plant Growth. Plant Physiol. 2004, 135, 1294–1304.
- Klose, H.; Günl, M.; Usadel, B.; Fischer, R.; Commandeur, U. Cell wall modification in tobacco by differential targeting of recombinant endoglucanase from Trichoderma reesei. BMC Plant Biol. 2015, 15, 54.
- Benedetti, M.; Vecchi, V.; Barera, S.; Dall’Osto, L. Biomass from microalgae: The potential of domestication towards sustainable biofactories. Microb. Cell Factories 2018, 17, 173.
- Imam, S.H.; Buchanan, M.J.; Shin, H.C.; Snell, W.J. The Chlamydomonas cell wall: Characterization of the wall framework. J. Cell Biol. 1985, 101, 1599–1607.
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289.
- Azhar, S.H.M.; Abdulla, R.; Jambo, S.A.; Marbawi, H.; Gansau, J.A.; Faik, A.A.M.; Rodrigues, K.F. Yeasts in sustainable bioethanol production: A review. Biochem. Biophys. Rep. 2017, 10, 52–61.
- Kainthola, J.; Kalamdhad, A.S.; Goud, V.V. A review on enhanced biogas production from anaerobic digestion of lignocellulosic biomass by different enhancement techniques. Process Biochem. 2019, 84, 81–90.
- Savatin, D.V.; Ferrari, S.; Sicilia, F.; De Lorenzo, G. Oligogalacturonide-Auxin Antagonism Does Not Require Posttranscriptional Gene Silencing or Stabilization of Auxin Response Repressors in Arabidopsis. Plant Physiol. 2011, 157, 1163–1174.
- Huot, B.; Yao, J.; Montgomery, B.L.; He, S.Y. Growth-defense tradeoffs in plants: A balancing act to optimize fitness. Mol. Plant 2014, 7, 1267–1287.