Ruminants such as cattle ferment feed to fulfill their energy requirement and gluconeogenesis in the liver is central to lactose synthesis in the mammary gland. During the peri-parturient period, there are fluctuations in the dry matter intake (DMI). A pronounced decline in DMI is observed in the last 10 days of parturition, followed by a marked increase afterwards, but this is presumably not sufficient to fulfill the increased nutrient and energetic demand of postpartum dairy cows [
20,
21,
22]. The post-parturient lactation peak is generally observed after 4 to 6 weeks, while the DMI peak arrives at 8 to 10 weeks. Hence, cows are in NEBAL at least 50 d postpartum. A recent study has related low prepartum DMI and energy balance with postpartum indigestion and metabolic disorders [
23]. The same group of researchers associated low prepartum DMI with postpartum reproductive disorders, while postpartum reproductive problems were associated with low postpartum DMI [
24]. Therefore, the phenomenon of adequate feed intake and body energy balance during the perinatal period is of immense importance for health and reproductive processes.
In addition to DMI, the body condition score (BCS) is widely used to assess the energy balance, health, and reproductive outcomes in postpartum dairy cows [
25]. High prepartum BCS is associated with increased risk of postpartum metabolic problems [
23], while other studies show that low prepartum BCS is responsible for prepartum metabolic and reproductive disorders [
26]. The reason for this conflict in studies is essentially due to the postpartum energetic metabolism changes related to fat mobilization [
27], where higher prepartum BCS (obesity) is associated with postpartum metabolic disorders and low reproductive efficiency [
28,
29]. Additionally, a prepartum BCS loss of 0.5 points or more could negatively affect perinatal blood Ca levels and predispose cows to the risk of ketosis and delayed conception [
30]. Furthermore, high prepartum BCS was associated with lower calf body weights [
31]. It is concluded that prepartum low BCS and over-conditioning (higher BCS) are both unfavorable for postpartum reproductive efficiency. The over-conditioned cows had lower mRNA levels of TNFα and higher mRNA levels of peroxisome proliferator-activated receptor gamma (PPARγ) in adipose tissues during postpartum, while the phosphorylated protein kinase B (AKT) pathway related to extensive metabolic shifts through downstream signaling of insulin in adipose tissue was also upregulated [
27]. The phenomenon of high inflammatory cytokine signaling and AKT signaling pathway upregulation synergistically enhance the energetic metabolism [
32,
33,
34]. It can be proposed that energy balance monitoring through serum metabolites can help to predict the postpartum nutritional physiology and its ultimate repercussions on reproductive performance [
30,
35].
Postpartum NEBAL is characterized by low blood glucose and insulin and high ketone bodies and non-esterified fatty acid (NEFA) concentrations [
36,
37,
38,
39]. However, NEBAL combined with nutritional metabolic diseases such as ketosis will delay the recovery of the uterus and normal reproductive process, leading to prolonged time to first service and increased calving intervals. In the presence of NEBAL, there is an increased incidence of nutritional metabolic disorders [
40]. The literature confirms that postpartum nutritional metabolic diseases are the major causes of post-parturient reproduction disorders in dairy cows [
41]. Nutritional metabolic diseases are complex “production diseases”, with a high incidence rate in modern dairy cows. In addition to the direct economic losses caused by reduced milk production, they can also have a long-term negative impact on the physiology of cows, especially their reproductive efficiency [
42,
43,
44]. A retrospective study of 7500 perinatal Holstein cows showed that within 21 d after parturition, about 1/3 of the cows presented with at least one subclinical or clinical metabolic disease. Furthermore, the 45 d fertilization rate decreased by 7%, milk yield decreased by 14%, and the culling rate increased from 22.6% (no clinical disease) to 35.7% (one clinical incidence) and 53.8% (more than three clinical incidences) for the cows suffering with metabolic diseases [
45]. Another study indicated a 26% incidence rate of ketosis during the observation period of 60 days postpartum [
46]. A study covering 12 countries from four continents found that the average subclinical ketosis prevalence in the first 21 days postpartum was 24.1%, ranging from 8.3% to 40.1% [
47]. Another study based on commercial dairy farm data reported a higher incidence of 56% for clinical or subclinical metabolic and reproductive diseases (ketosis, hypocalcemia, metritis, and mastitis) in the first 3 weeks postpartum [
48].
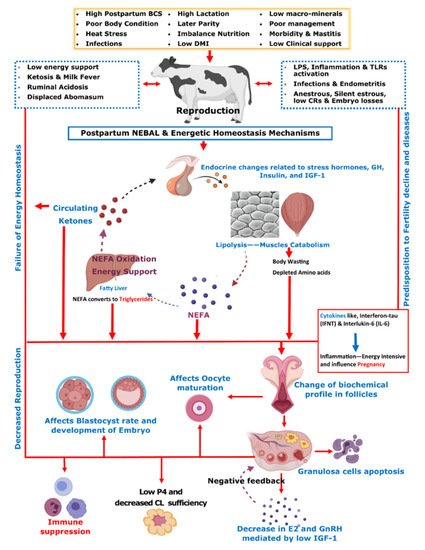
Initial post-parturient lactation is maintained at the expense of a decline in reproductive performance [
10]. The mechanism involving underlying NEBAL can be explained by low insulin levels causing an abundance of growth hormones (GHs), which in turn mobilize NEFAs. However, at the same time, there is a decrease in hepatic GH receptor abundance, preventing negative feedback through IGF-1 [
38,
49], while the presence of NEFAs is consistently behind the low insulin via catecholamines [
50]. As lipolysis helps the lactation demand, this is also the major cause of low BCS in postpartum cows. Thus, postpartum BCS is an indirect measurement of fat metabolism and correlates with the energy balance of cows [
51]. These aforementioned metabolic alterations mediated by complex endocrine changes have further consequences for ovulation, oocytes, and the corpus luteum [
38,
49,
52,
53,
54]. Further connections of these changes with reproductive performance include low concentrations of insulin and insulin growth factor 1 (IGF-1) causing follicular biochemical changes in ovaries and thus influencing luteinizing hormone (LH) and estradiol (E2) secretion [
52,
53,
55]. This decrease in LH and E2 secretions in turn ultimately leads to delayed resumption of the estrus cycle [
56]. On the other hand, NEBAL is also shown to be associated with low progesterone concentrations, which negatively influence the early pregnancy outcome [
57,
58]. Carrying the discussion further, a high concentration of blood NEFAs is shown to be negatively associated with the developmental competence of fertilized oocytes [
59,
60]. Generally, an increase above 0.55 mmol/L of plasma NEFA levels is regarded as a manifestation of serious postpartum NEBAL [
61,
62]. Postpartum NEBAL and high NEFA concentrations have shown evidence for higher levels of inflammation characterized by cytokines and Toll-like receptor (TLR) expression leading to alterations in uterine functions [
63,
64,
65]. Furthermore, NEFA exerts cytotoxic effects at cellular levels and is attributed to immunosuppression [
66,
67]. As NEBAL in itself possesses implications for early reproductive recovery, it is also obvious that postpartum NEBAL can be regarded as a root cause of various postpartum production diseases. Based on the discussions in this review,
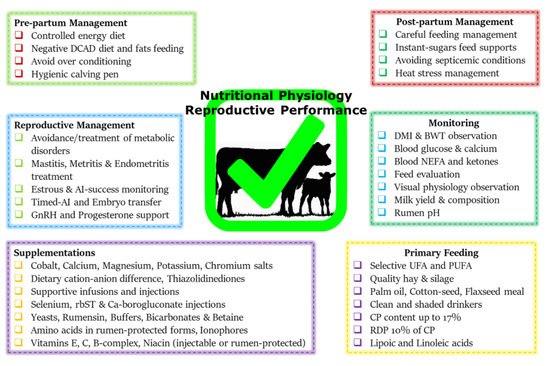
Figure 1 summarizes various metabolic and endocrine mechanisms contributing to the decline in post-parturient reproductive efficiency of dairy cows. A score of mitigation strategies have been advised to minimize extreme postpartum NEBAL incidence. Dry cow management should be oriented at the feeding of energy-rich diets over the duration of 3–4 weeks prepartum, in order to support fetal growth, the decline in DMI, and peri-parturient events [
68]. This phenomenon of increasing the energy content of the diet can also be supported by the facts of decreased DMI intake and rumination time during the last 3 weeks of gestation [
21,
24,
69]. However, over-conditioning of prepartum cows is not desirable and leads to postpartum metabolic disorders [
41,
70]. A prepartum controlled energy diet near the calculated requirements essentially averted the risk of postpartum NEBAL [
70,
71]. An increased energy prepartum diet is shown to enhance fat accumulation, decrease DMI, and increase the risk of health problems in postpartum dairy cows [
68,
70,
72]. Similarly, others have shown that an increased energy diet prepartum can lead to increased NEFA mobilization and triglyceride (TG) accumulation in the liver of postpartum cows [
73], while a controlled energy diet prepartum is shown to improve the immune function of postpartum dairy cows [
74]. Therefore, careful feeding management through monitoring of feed energy content and BCS assessment of prepartum cows constitute an essential key to perinatal transition success.