Herein, state-of-the-art research advances in South Korea regarding the development of chemical sensing materials and fully integrated Internet of Things (IoT) sensing platforms were comprehensively reviewed for verifying the applicability of such sensing systems in point-of-care testing (POCT). Various organic/inorganic nanomaterials were synthesized and characterized to understand their fundamental chemical sensing mechanisms upon exposure to target analytes. Moreover, the applicability of nanomaterials integrated with IoT-based signal transducers for the real-time and on-site analysis of chemical species was verified.
- nanostructure
- IoT
- POCT
- gas sensor
- ion sensor
- biosensor
1. Introduction
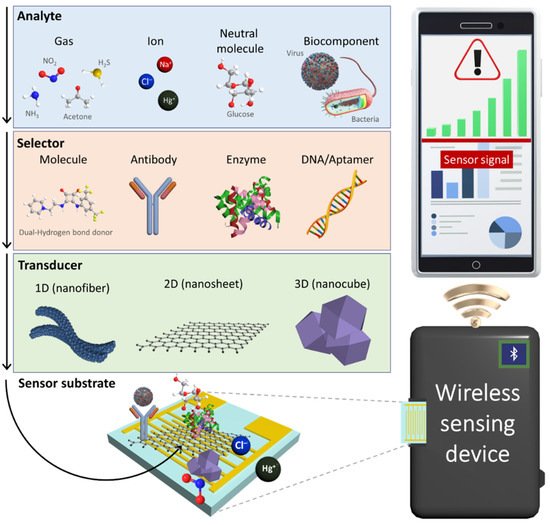
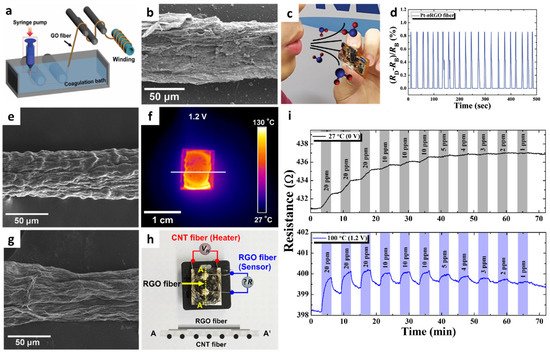
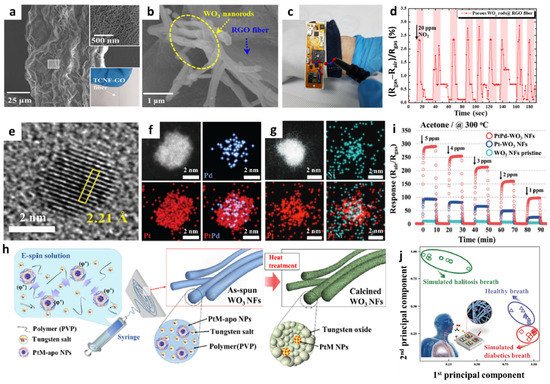
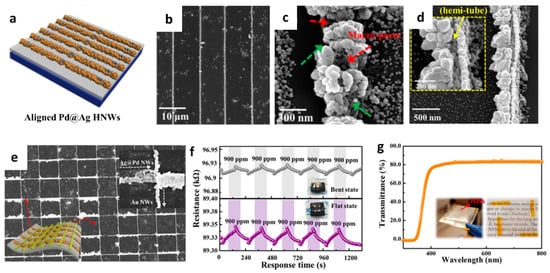
2. Gas Sensors
3. Ion Sensors
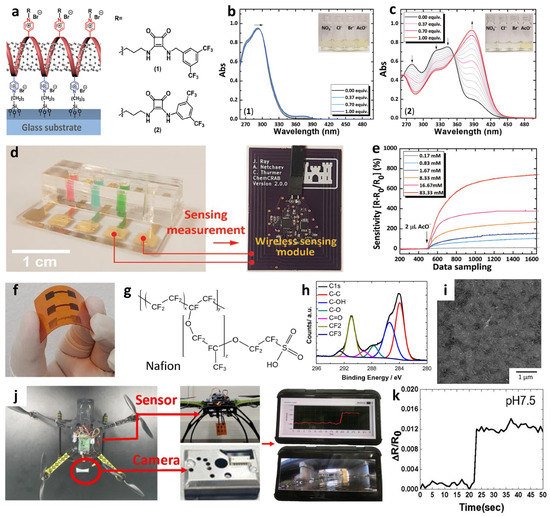
| Material | Response Definition | Response | Detection Limit | Testing Ambient | Target Ions | Applications | Ref. |
|---|---|---|---|---|---|---|---|
| SWCNT-P4VP-Squaramide | ΔR/R0 (%) | 120.27% @ 16.7 mM | 1.7 mM | Acetonitrile | CH3COO− | IoT sensor | [77] |
| SWCNT-Nafion | ΔR/R0 | ~0.2 @ pH 12 | - | H2O | H+ | IoT sensor | [82] |
| SWCNT-P4VP-Thiourea | ΔR/R0 (%) | 101.9% @ 16.7 mM | 0.17 mM | Acetonitrile | CH3COO− | - | [85] |
| SWCNT-P4VP-Croconamide | ΔR/R0 (%) | 140.91% @ 83.33 mM | 0.17 mM | Acetonitrile | CH3COO− | - | [86] |
| Material | Response | Dynamic Range | Testing Ambient | Target Ions | Response/Recovery Time | Applications | Ref. |
|---|---|---|---|---|---|---|---|
| PCSC-coated CFT | 60.7 ± 1.5 mV log[Na+]−1 54.8 ± 0.6 mV log[K+]−1 |
10−1–10−4 M | Body fluid (sweat) | Na+, K+ | 10–20 s | IoT wearable sensor | [94] |
| PCSC-coated CFT | 58.28 mV/pH | pH 3.89–10.09 | Body fluid (sweat) | H+ | 5 s | IoT wearable sensor | [95] |
| WO3 NFs/ CI-TPES |
−377.5 mV/pH (With differential amp.) |
pH 6.90–8.94 | Artificial seawater | H+ | - | Ocean acidification monitoring | [96] |
| Defect-free exfoliated graphene | 54.0 mV log [Na+]−1 | 10−1–10−4 M | Body fluid (sweat) | Na+ | 3.6 s | IoT wearable sensor | [97] |
| MWCNTs– MXene (Ti3C2TX) |
63 mV log [K+]−1 | 1–32 mM | Body fluid (sweat) | K+ | 2 s | IoT wearable sensor | [98] |
4. Biosensors
This entry is adapted from the peer-reviewed paper 10.3390/s22020610
References
- Lim, J.W.; Kim, T.-Y.; Woo, M.-A. Trends in sensor development toward next-generation point-of-care testing for mercury. Biosens. Bioelectron. 2021, 183, 113228.
- Yoon, J.-W.; Lee, J.-H. Toward breath analysis on a chip for disease diagnosis using semiconductor-based chemiresistors: Recent progress and future perspectives. Lab Chip 2017, 17, 3537–3557.
- Sempionatto, J.R.; Jeerapan, I.; Krishnan, S.; Wang, J. Wearable chemical sensors: Emerging systems for on-body analytical chemistry. Anal. Chem. 2019, 92, 378–396.
- Justino, C.I.; Freitas, A.C.; Pereira, R.; Duarte, A.C.; Santos, T.A.R. Recent developments in recognition elements for chemical sensors and biosensors. TrAC Trends Anal. Chem. 2015, 68, 2–17.
- Majhi, S.M.; Mirzaei, A.; Kim, H.W.; Kim, S.S.; Kim, T.W. Recent advances in energy-saving chemiresistive gas sensors: A review. Nano Energy 2021, 79, 105369.
- Choi, S.-J.; Kim, I.-D. Recent developments in 2D nanomaterials for chemiresistive-type gas sensors. Electron. Mater. Lett. 2018, 14, 221–260.
- Yang, C.; Denno, M.E.; Pyakurel, P.; Venton, B.J. Recent trends in carbon nanomaterial-based electrochemical sensors for biomolecules: A review. Anal. Chim. Acta 2015, 887, 17–37.
- Yao, S.; Swetha, P.; Zhu, Y. Nanomaterial-enabled wearable sensors for healthcare. Adv. Healthc. Mater. 2018, 7, 1700889.
- Kim, S.-J.; Choi, S.-J.; Jang, J.-S.; Cho, H.-J.; Kim, I.-D. Innovative nanosensor for disease diagnosis. Acc. Chem. Res. 2017, 50, 1587–1596.
- Wang, Z.; Hu, T.; Liang, R.; Wei, M. Application of zero-dimensional nanomaterials in biosensing. Front. Chem. 2020, 8, 320.
- Choi, S.J.; Persano, L.; Camposeo, A.; Jang, J.S.; Koo, W.T.; Kim, S.J.; Cho, H.J.; Kim, I.D.; Pisignano, D. Electrospun nanostructures for high performance chemiresistive and optical sensors. Macromol. Mater. Eng. 2017, 302, 1600569.
- Choi, S.-J.; Kim, I.-D.; Park, H.J. 2D layered Mn and Ru oxide nanosheets for real-time breath humidity monitoring. Appl. Surf. Sci. 2022, 573, 151481.
- Liu, X.; Ma, T.; Pinna, N.; Zhang, J. Two-dimensional nanostructured materials for gas sensing. Adv. Funct. Mater. 2017, 27, 1702168.
- Jeong, J.-M.; Yang, M.; Kim, D.S.; Lee, T.J.; Choi, B.G. High performance electrochemical glucose sensor based on three-dimensional MoS2/graphene aerogel. J. Colloid Interface Sci. 2017, 506, 379–385.
- Choi, S.-J.; Choi, H.-J.; Koo, W.-T.; Huh, D.; Lee, H.; Kim, I.-D. Metal–organic framework-templated PdO-Co3O4 nanocubes functionalized by SWCNTs: Improved NO2 reaction kinetics on flexible heating film. ACS Appl. Mater. Interfaces 2017, 9, 40593–40603.
- Kwon, O.S.; Song, H.S.; Park, T.H.; Jang, J. Conducting nanomaterial sensor using natural receptors. Chem. Rev. 2018, 119, 36–93.
- Fang, Y.; Deng, Y.; Dehaen, W. Tailoring pillararene-based receptors for specific metal ion binding: From recognition to supramolecular assembly. Coord. Chem. Rev. 2020, 415, 213313.
- Durkin, T.J.; Barua, B.; Savagatrup, S. Rapid detection of sepsis: Recent advances in biomarker sensing platforms. ACS Omega 2021, 6, 31390–31395.
- Kucherenko, I.; Soldatkin, O.; Dzyadevych, S.; Soldatkin, A. Electrochemical biosensors based on multienzyme systems: Main groups, advantages and limitations—A review. Anal. Chim. Acta 2020, 1111, 114–131.
- Li, F.; Yu, Z.; Han, X.; Lai, R.Y. Electrochemical aptamer-based sensors for food and water analysis: A review. Anal. Chim. Acta 2019, 1051, 1–23.
- Naresh, V.; Lee, N. A review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors 2021, 21, 1109.
- Mohammadniaei, M.; Nguyen, H.V.; Tieu, M.V.; Lee, M.-H. 2D materials in development of electrochemical point-of-care cancer screening devices. Micromachines 2019, 10, 662.
- Sivakumar, R.; Lee, N.Y. Recent progress in smartphone-based techniques for food safety and the detection of heavy metal ions in environmental water. Chemosphere 2021, 275, 130096.
- Zhao, J.; Lin, Y.; Wu, J.; Nyein, H.Y.Y.; Bariya, M.; Tai, L.-C.; Chao, M.; Ji, W.; Zhang, G.; Fan, Z. A fully integrated and self-powered smartwatch for continuous sweat glucose monitoring. ACS Sens. 2019, 4, 1925–1933.
- Bandodkar, A.J.; Gutruf, P.; Choi, J.; Lee, K.; Sekine, Y.; Reeder, J.T.; Jeang, W.J.; Aranyosi, A.J.; Lee, S.P.; Model, J.B. Battery-free, skin-interfaced microfluidic/electronic systems for simultaneous electrochemical, colorimetric, and volumetric analysis of sweat. Sci. Adv. 2019, 5, eaav3294.
- Sempionatto, J.R.; Nakagawa, T.; Pavinatto, A.; Mensah, S.T.; Imani, S.; Mercier, P.; Wang, J. Eyeglasses based wireless electrolyte and metabolite sensor platform. Lab Chip 2017, 17, 1834–1842.
- Park, J.; Kim, J.; Kim, S.-Y.; Cheong, W.H.; Jang, J.; Park, Y.-G.; Na, K.; Kim, Y.-T.; Heo, J.H.; Lee, C.Y. Soft, smart contact lenses with integrations of wireless circuits, glucose sensors, and displays. Sci. Adv. 2018, 4, eaap9841.
- Feng, S.B.; Farha, F.; Li, Q.J.; Wan, Y.L.; Xu, Y.; Zhang, T.; Ning, H.S. Review on Smart gas sensing technology. Sensors 2019, 19, 3790.
- Kim, E.; Lee, S.; Kim, J.H.; Kim, C.; Byun, Y.T.; Kim, H.S.; Lee, T. Pattern recognition for selective odor detection with gas sensor arrays. Sensors 2012, 12, 16262–16273.
- Speizer, F.E.; Ferris, B.; Bishop, Y.M.M.; Spengler, J. Respiratory-disease rates and pulmonary-function in children associated with NO2 exposure. Am. Rev. Respir. Dis 1980, 121, 3–10.
- Das, S.; Pal, M. Non-invasive monitoring of human health by exhaled breath analysis: A comprehensive review. J. Electrochem. Soc. 2020, 167, 037562.
- Tassopoulos, C.N.; Barnett, D.; Russell Fraser, T. Breath-acetone and blood-sugar measurements in diabetes. Lancet 1969, 293, 1282–1286.
- Zhou, X.; Xue, Z.; Chen, X.; Huang, C.; Bai, W.; Lu, Z.; Wang, T. Nanomaterial-based gas sensors used for breath diagnosis. J. Mater. Chem. B 2020, 8, 3231–3248.
- Baharuddin, A.A.; Ang, B.C.; Haseeb, A.; Wong, Y.C.; Wong, Y.H. Advances in chemiresistive sensors for acetone gas detection. Mater. Sci. Semicond. Process. 2019, 103, 104616.
- Wang, H.; Ma, J.; Zhang, J.; Feng, Y.; Vijjapu, M.T.; Yuvaraja, S.; Surya, S.G.; Salama, K.N.; Dong, C.; Wang, Y.; et al. Gas sensing materials roadmap. J. Phys. Condens. Matter 2021, 33, 303001.
- Zhao, S.; Shen, Y.; Yan, X.; Zhou, P.; Yin, Y.; Lu, R.; Han, C.; Cui, B.; Wei, D. Complex-surfactant-assisted hydrothermal synthesis of one-dimensional ZnO nanorods for high-performance ethanol gas sensor. Sens. Actuators B Chem. 2019, 286, 501–511.
- Al-Hazeem, N.Z.; Ahmed, N.M.; Matjafri, M.Z.; Bououdina, M. Hydrogen gas sensor based on nanofibers TiO2-PVP thin film at room temperature prepared by electrospinning. Microsyst. Technol. 2021, 27, 293–299.
- Li, P.; Zhang, Z.; Zhuang, Z.; Guo, J.; Fang, Z.; Fereja, S.L.; Chen, W. Pd-doping-induced oxygen vacancies in one-dimensional tungsten oxide nanowires for enhanced acetone gas sensing. Anal. Chem. 2021, 93, 7465–7472.
- Wang, Z.; Zhu, L.; Sun, S.; Wang, J.; Yan, W. One-dimensional nanomaterials in resistive gas sensor: From material design to application. Chemosensors 2021, 9, 198.
- Moon, D.-B.; Bag, A.; Lee, H.-B.; Meeseepong, M.; Lee, D.-H.; Lee, N.-E. A stretchable, room-temperature operable, chemiresistive gas sensor using nanohybrids of reduced graphene oxide and zinc oxide nanorods. Sens. Actuators B Chem. 2021, 345, 130373.
- Shaalan, N.M.; Yamazaki, T.; Kikuta, T. Effect of micro-electrode geometry on NO2 gas-sensing characteristics of one-dimensional tin dioxide nanostructure microsensors. Sens. Actuators B Chem. 2011, 156, 784–790.
- Moon, J.; Park, J.-A.; Lee, S.-J.; Zyung, T.; Kim, I.-D. Pd-doped TiO2 nanofiber networks for gas sensor applications. Sens. Actuators B Chem. 2010, 149, 301–305.
- Feng, C.; Kou, X.; Liao, X.; Sun, Y.; Lu, G. One-dimensional Cr-doped NiO nanostructures serving as a highly sensitive gas sensor for trace xylene detection. RSC Adv. 2017, 7, 41105–41110.
- Li, W.; Chen, R.; Qi, W.; Cai, L.; Sun, Y.; Sun, M.; Li, C.; Yang, X.; Xiang, L.; Xie, D.; et al. Reduced graphene oxide/mesoporous ZnO NSs hybrid fibers for flexible, stretchable, twisted, and wearable NO2 e-textile gas sensor. ACS Sens. 2019, 4, 2809–2818.
- Xu, Z.; Gao, C. Graphene fiber: A new trend in carbon fibers. Mater. Today 2015, 18, 480–492.
- Choi, S.-J.; Yu, H.; Jang, J.-S.; Kim, M.-H.; Kim, S.-J.; Jeong, H.S.; Kim, I.-D. Nitrogen-doped single graphene fiber with platinum water dissociation catalyst for wearable humidity sensor. Small 2018, 14, 1703934.
- Cong, H.-P.; Ren, X.-C.; Wang, P.; Yu, S.-H. Wet-spinning assembly of continuous, neat and macroscopic graphene fibers. Sci. Rep. 2012, 2, 613.
- Du, D.; Li, P.; Ouyang, J. Nitrogen-doped reduced graphene oxide prepared by simultaneous thermal reduction and nitrogen doping of graphene oxide in air and its application as an electrocatalyst. ACS Appl. Mater. Interfaces 2015, 7, 26952–26958.
- Jutz, G.; Van Rijn, P.; Santos Miranda, B.; Böker, A. Ferritin: A versatile building block for bionanotechnology. Chem. Rev. 2015, 115, 1653–1701.
- Fajin, J.L.; Bruix, A.; Cordeiro, M.N.; Gomes, J.R.; Illas, F. Density functional theory model study of size and structure effects on water dissociation by platinum nanoparticles. J. Chem. Phys. 2012, 137, 034701.
- Choi, S.J.; Lee, D.M.; Yu, H.; Jang, J.S.; Kim, M.H.; Kang, J.Y.; Jeong, H.S.; Kim, I.D. All-carbon fiber-based chemical sensor: Improved reversible NO2 reaction kinetics. Sens. Actuators B Chem. 2019, 290, 293–301.
- Choi, S.J.; Kim, S.J.; Jang, J.S.; Lee, J.H.; Kim, I.D. Silver nanowire embedded colorless polyimide heater for wearable chemical sensors: Improved reversible reaction kinetics of optically reduced graphene oxide. Small 2016, 12, 5826–5835.
- Eom, W.; Jang, J.S.; Lee, S.H.; Lee, E.; Jeong, W.; Kim, I.D.; Choi, S.J.; Han, T.H. Effect of metal/metal oxide catalysts on graphene fiber for improved NO2 sensing. Sens. Actuators B Chem. 2021, 344.
- Liu, J.; Zhang, L.; Cheng, B.; Fan, J.; Yu, J. A high-response formaldehyde sensor based on fibrous Ag-ZnO/In2O3 with multi-level heterojunctions. J. Hazard. Mater. 2021, 413, 125352.
- Liu, A.; Lv, S.; Jiang, L.; Liu, F.; Zhao, L.; Wang, J.; Hu, X.; Yang, Z.; He, J.; Wang, C.; et al. The gas sensor utilizing polyaniline/MoS2 nanosheets/SnO2 nanotubes for the room temperature detection of ammonia. Sens. Actuators B Chem. 2021, 332, 129444.
- Mishra, R.K.; Murali, G.; Kim, T.-H.; Kim, J.H.; Lim, Y.J.; Kim, B.-S.; Sahay, P.P.; Lee, S.H. Nanocube In2O3@RGO heterostructure based gas sensor for acetone and formaldehyde detection. RSC Adv. 2017, 7, 38714–38724.
- Jang, J.S.; Yu, H.; Choi, S.J.; Koo, W.T.; Lee, J.; Kim, D.H.; Kang, J.Y.; Jeong, Y.J.; Jeong, H.; Kim, I.D. Heterogeneous metal oxide-graphene thorn-bush single fiber as a freestanding chemiresistor. ACS Appl. Mater. Interfaces 2019, 11, 10208–10217.
- Cho, S.Y.; Yu, H.; Choi, J.; Kang, H.; Park, S.; Jang, J.S.; Hong, H.J.; Kim, I.D.; Lee, S.K.; Jeong, H.S.; et al. Continuous meter-scale synthesis of weavable tunicate cellulose/carbon nanotube fibers for high-performance wearable sensors. ACS Nano 2019, 13, 9332–9341.
- Cheng, Q.; Ye, D.; Chang, C.; Zhang, L. Facile fabrication of superhydrophilic membranes consisted of fibrous tunicate cellulose nanocrystals for highly efficient oil/water separation. J. Membr. Sci. 2017, 525, 1–8.
- Nakate, U.T.; Ahmad, R.; Patil, P.; Wang, Y.; Bhat, K.S.; Mahmoudi, T.; Yu, Y.T.; Suh, E.-K.; Hahn, Y.-B. Improved selectivity and low concentration hydrogen gas sensor application of Pd sensitized heterojunction n-ZnO/p-NiO nanostructures. J. Alloys Compd. 2019, 797, 456–464.
- Kim, S.J.; Choi, S.J.; Jang, J.S.; Cho, H.J.; Koo, W.T.; Tuller, H.L.; Kim, I.D. Exceptional high-performance of pt-based bimetallic catalysts for exclusive detection of exhaled biomarkers. Adv. Mater. 2017, 29, 1700737.
- Zou, Y.; Chen, S.; Sun, J.; Liu, J.; Che, Y.; Liu, X.; Zhang, J.; Yang, D. Highly efficient gas sensor using a hollow SnO2 microfiber for triethylamine detection. ACS Sens. 2017, 2, 897–902.
- Cha, J.-H.; Choi, S.-J.; Yu, S.; Kim, I.-D. 2D WS2-edge functionalized multi-channel carbon nanofibers: Effect of WS2 edge-abundant structure on room temperature NO2 sensing. J. Mater. Chem. A 2017, 5, 8725–8732.
- Han, D.; Ji, Y.; Gu, F.; Wang, Z. Cobalt oxide nanorods with special pore structure for enhanced ethanol sensing performance. J. Colloid Interface Sci. 2018, 531, 320–330.
- Jang, J.S.; Qiao, S.; Choi, S.J.; Jha, G.; Ogata, A.F.; Koo, W.T.; Kim, D.H.; Kim, I.D.; Penner, R.M. Hollow Pd–Ag composite nanowires for fast responding and transparent hydrogen sensors. ACS Appl. Mater. Interfaces 2017, 9, 39464–39474.
- Jo, S.; Sung, D.; Kim, S.; Koo, J. A review of wearable biosensors for sweat analysis. Biomed. Eng. Lett. 2021, 11, 117–129.
- Kaisti, M.; Boeva, Z.; Koskinen, J.; Nieminen, S.; Bobacka, J.; Levon, K. Hand-held transistor based electrical and multiplexed chemical sensing system. Acs Sens. 2016, 1, 1423–1431.
- Kang, S.G.; Song, M.S.; Kim, J.W.; Lee, J.W.; Kim, J. Near-field communication in biomedical applications. Sensors 2021, 21, 703.
- Baker, L.B.; Model, J.B.; Barnes, K.A.; Anderson, M.L.; Lee, S.P.; Lee, K.A.; Brown, S.D.; Reimel, A.J.; Roberts, T.J.; Nuccio, R.P. Skin-interfaced microfluidic system with personalized sweating rate and sweat chloride analytics for sports science applications. Sci. Adv. 2020, 6, eabe3929.
- Eiteman, M.A.; Altman, E. Overcoming acetate in Escherichia coli recombinant protein fermentations. Trends Biotechnol. 2006, 24, 530–536.
- Wolfe, A.J. The acetate switch. Microbiol. Mol. Biol. Rev. 2005, 69, 12–50.
- Dittrich, C.R.; Vadali, R.V.; Bennett, G.N.; San, K.Y. Redistribution of metabolic fluxes in the central aerobic metabolic pathway of E. coli mutant strains with deletion of the ackA-pta and poxB pathways for the synthesis of isoamyl acetate. Biotechnol. Prog. 2005, 21, 627–631.
- Xu, G.; Cheng, C.; Yuan, W.; Liu, Z.Y.; Zhu, L.H.; Li, X.T.; Lu, Y.L.; Chen, Z.T.; Liu, J.L.; Cui, Z.; et al. Smartphone-based battery-free and flexible electrochemical patch for calcium and chloride ions detections in biofluids. Sens. Actuators B Chem. 2019, 297, 126743.
- Choi, D.-H.; Li, Y.; Cutting, G.R.; Searson, P.C. A wearable potentiometric sensor with integrated salt bridge for sweat chloride measurement. Sens. Actuators B Chem. 2017, 250, 673–678.
- Chen, L.J.; Berry, S.N.; Wu, X.; Howe, E.N.W.; Gale, P.A. Advances in anion receptor chemistry. Chem 2020, 6, 61–141.
- Amendola, V.; Bergamaschi, G.; Boiocchi, M.; Fabbrizzi, L.; Milani, M. The squaramide versus urea contest for anion recognition. Chem.-Eur. J. 2010, 16, 4368–4380.
- Choi, S.J.; Yoon, B.; Ray, J.D.; Netchaev, A.; Moores, L.C.; Swager, T.M. Chemiresistors for the real-time wireless detection of anions. Adv. Funct. Mater. 2020, 30, 1907087.
- Manjakkal, L.; Dang, W.T.; Yogeswaran, N.; Dahiya, R. Textile-based potentiometric electrochemical pH sensor for wearable applications. Biosensors 2019, 9, 14.
- Promphet, N.; Rattanawaleedirojn, P.; Siralertmukul, K.; Soatthiyanon, N.; Potiyaraj, P.; Thanawattano, C.; Hinestroza, J.P.; Rodthongkum, N. Non-invasive textile based colorimetric sensor for the simultaneous detection of sweat pH and lactate. Talanta 2019, 192, 424–430.
- Qin, Y.H.; Kwon, H.J.; Howlader, M.M.R.; Deen, M.J. Microfabricated electrochemical pH and free chlorine sensors for water quality monitoring: Recent advances and research challenges. RSC Adv. 2015, 5, 69086–69109.
- Dore, J.E.; Lukas, R.; Sadler, D.W.; Church, M.J.; Karl, D.M. Physical and biogeochemical modulation of ocean acidification in the central North Pacific. Proc. Natl. Acad. Sci. USA 2009, 106, 12235–12240.
- Jeon, J.Y.; Kang, B.C.; Ha, T.J. Flexible pH sensors based on printed nanocomposites of single-wall carbon nanotubes and nafion. Appl. Surf. Sci. 2020, 514, 145956.
- Liu, Y.-L.; Su, Y.-H.; Chang, C.-M.; Wang, D.-M.; Lai, J.-Y. Preparation and applications of Nafion-functionalized multiwalled carbon nanotubes for proton exchange membrane fuel cells. J. Mater. Chem. 2010, 20, 4409–4416.
- Siu, A.; Schmeisser, J.; Holdcroft, S. Effect of water on the low temperature conductivity of polymer electrolytes. J. Phys. Chem. B 2006, 110, 6072–6080.
- Choi, S.J.; Yoon, B.; Lin, S.B.; Swager, T.M. Functional single-walled carbon nanotubes for anion sensing. ACS Appl. Mater. Interfaces 2020, 12, 28375–28382.
- Yoon, B.; Choi, S.J. Selective acetate recognition and sensing using SWCNTs functionalized with croconamides. Sens. Actuators B Chem. 2021, 346, 130461.
- Parrilla, M.; Cuartero, M.; Crespo, G.A. Wearable potentiometric ion sensors. TrAC Trend Anal. Chem. 2019, 110, 303–320.
- Cuartero, M.; Parrilla, M.; Crespo, G.A. Wearable potentiometric sensors for medical applications. Sensors 2019, 19, 363.
- Bandodkar, A.J.; Wang, J. Non-invasive wearable electrochemical sensors: A review. Trends Biotechnol. 2014, 32, 363–371.
- Xu, J.N.; Zhang, Z.; Gan, S.Y.; Gao, H.; Kong, H.J.; Song, Z.Q.; Ge, X.M.; Bao, Y.; Niu, L. Highly stretchable fiber-based potentiometric ion sensors for multichannel real-time analysis of human sweat. ACS Sens. 2020, 5, 2834–2842.
- An, Q.B.; Gan, S.Y.; Xu, J.N.; Bao, Y.; Wu, T.S.; Kong, H.J.; Zhong, L.J.; Ma, Y.M.; Song, Z.Q.; Niu, L. A multichannel electrochemical all-solid-state wearable potentiometric sensor for real-time sweat ion monitoring. Electrochem. Commun. 2019, 107, 106553.
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.V.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509.
- Wu, W.W.; Haick, H. Materials and wearable devices for autonomous monitoring of physiological markers. Adv. Mater. 2018, 30.
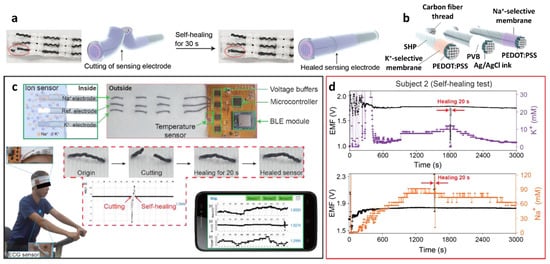
- Yoon, J.H.; Kim, S.M.; Eom, Y.; Koo, J.M.; Cho, H.W.; Lee, T.J.; Lee, K.G.; Park, H.J.; Kim, Y.K.; Yoo, H.J.; et al. Extremely fast self-healable bio-based supramolecular polymer for wearable real-time sweat-monitoring sensor. ACS Appl. Mater. Interfaces 2019, 11, 46165–46175.
- Yoon, J.H.; Kim, S.M.; Park, H.J.; Kim, Y.K.; Oh, D.X.; Cho, H.W.; Lee, K.G.; Hwang, S.Y.; Park, J.; Choi, B.G. Highly self-healable and flexible cable-type pH sensors for real-time monitoring of human fluids. Biosens. Bioelectron. 2020, 150, 111946.
- Choi, S.J.; Savagatrup, S.; Kim, Y.; Lang, J.H.; Swager, T.M. Precision pH sensor based on WO3 nanofiber-polymer composites and differential amplification. ACS Sens. 2019, 4, 2593–2598.
- Kim, D.S.; Jeong, J.M.; Park, H.J.; Kim, Y.K.; Lee, K.G.; Choi, B.G. Highly concentrated, conductive, defect-free graphene ink for screen-printed sensor application. Nano-Micro Lett. 2021, 13, 87.
- Zhang, S.P.; Abu Zahed, M.; Sharifuzzaman, M.; Yoon, S.; Hui, X.; Barman, S.C.; Sharma, S.; Yoon, H.S.; Park, C.; Park, J.Y. A wearable battery-free wireless and skin-interfaced microfluidics integrated electrochemical sensing patch for on-site biomarkers monitoring in human perspiration. Biosens. Bioelectron. 2021, 175, 112844.
- Kim, D.H.; Lu, N.S.; Ma, R.; Kim, Y.S.; Kim, R.H.; Wang, S.D.; Wu, J.; Won, S.M.; Tao, H.; Islam, A.; et al. Epidermal electronics. Science 2011, 333, 838–843.
- Yeo, W.H.; Kim, Y.S.; Lee, J.; Ameen, A.; Shi, L.K.; Li, M.; Wang, S.D.; Ma, R.; Jin, S.H.; Kang, Z.; et al. Multifunctional epidermal electronics printed directly onto the skin. Adv. Mater. 2013, 25, 2773–2778.
- Trung, T.Q.; Lee, N.E. Flexible and stretchable physical sensor integrated platforms for wearable human-activity monitoring and personal healthcare. Adv. Mater. 2016, 28, 4338–4372.
- Huynh, T.P.; Sonar, P.; Haick, H. Advanced materials for use in soft self-healing devices. Adv. Mater. 2017, 29, 1604973.
- Thakur, V.K.; Kessler, M.R. Self-healing polymer nanocomposite materials: A review. Polymer 2015, 69, 369–383.
- Li, J.J.; Geng, L.F.; Wang, G.; Chu, H.J.; Wei, H.L. Self-healable gels for use in wearable devices. Chem. Mater. 2017, 29, 8932–8952.
- Luo, C.S.; Wan, P.B.; Yang, H.; Shah, S.A.A.; Chen, X.D. Healable transparent electronic devices. Adv. Funct. Mater. 2017, 27, 1606339.
- Chen, D.D.; Wang, D.R.; Yang, Y.; Huang, Q.Y.; Zhu, S.J.; Zheng, Z.J. Self-healing materials for next-generation energy harvesting and storage devices. Adv. Energy Mater. 2017, 7, 1700890.
- Zou, Z.N.; Zhu, C.P.; Li, Y.; Lei, X.F.; Zhang, W.; Xiao, J.L. Rehealable, fully recyclable, and malleable electronic skin enabled by dynamic covalent thermoset nanocomposite. Sci. Adv. 2018, 4, eaaq0508.
- Kim, J.; Campbell, A.S.; de Avila, B.E.F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406.
- Lee, H.; Hong, Y.J.; Baik, S.; Hyeon, T.; Kim, D.H. Enzyme-based glucose sensor: From invasive to wearable device. Adv. Healthc. Mater. 2018, 7, 1701150.
- Shin, H.; Seo, H.; Chung, W.G.; Joo, B.J.; Jang, J.; Park, J.U. Recent progress on wearable point-of-care devices for ocular systems. Lab Chip 2021, 21, 1269–1286.
- Tang, L.; Chang, S.J.; Chen, C.J.; Liu, J.T. Non-invasive blood glucose monitoring technology: A review. Sensors 2020, 20, 6925.
- Teymourian, H.; Barfidokht, A.; Wang, J. Electrochemical glucose sensors in diabetes management: An updated review (2010–2020). Chem. Soc. Rev. 2020, 49, 7671–7709.
- Nichols, S.P.; Koh, A.; Storm, W.L.; Shin, J.H.; Schoenfisch, M.H. Biocompatible materials for continuous glucose monitoring devices. Chem. Rev. 2013, 113, 2528–2549.
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; Fernandes, J.D.D.; Ohlrogge, A.W.; Malanda, B. IDF diabetes atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281.
- Harding, J.L.; Pavkov, M.E.; Magliano, D.J.; Shaw, J.E.; Gregg, E.W. Global trends in diabetes complications: A review of current evidence. Diabetologia 2019, 62, 3–16.
- Heller, A.; Feldman, B. Electrochemical glucose sensors and their applications in diabetes management. Chem. Rev. 2008, 108, 2482–2505.
- Ciobanu, M.; Taylor, D.E.; Wilburn, J.P.; Cliffel, D.E. Glucose and lactate biosensors for scanning electrochemical microscopy imaging of single live cells. Anal. Chem. 2008, 80, 2717–2727.
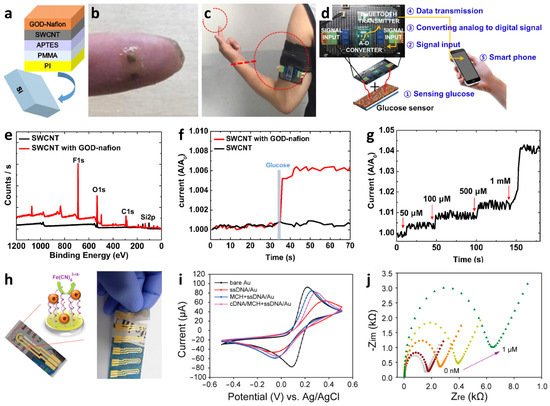
- Soylemez, S.; Yoon, B.; Toppare, L.; Swager, T.M. Quaternized polymer-single-walled carbon nanotube scaffolds for a chemiresistive glucose sensor. ACS Sens. 2017, 2, 1123–1127.
- Fang, L.; Liang, B.; Yang, G.; Hu, Y.C.; Zhu, Q.; Ye, X.S. A needle-type glucose biosensor based on PANI nanofibers and PU/E-PU membrane for long-term invasive continuous monitoring. Biosens. Bioelectron. 2017, 97, 196–202.
- Chen, D.J.; Wang, C.; Chen, W.; Chen, Y.Q.; Zhang, J.X.J. PVDF-Nafion nanomembranes coated microneedles for in vivo transcutaneous implantable glucose sensing. Biosens. Bioelectron. 2015, 74, 1047–1052.
- Wang, L.Y.; Xie, S.L.; Wang, Z.Y.; Liu, F.; Yang, Y.F.; Tang, C.Q.; Wu, X.Y.; Liu, P.; Li, Y.J.; Saiyin, H.; et al. Functionalized helical fibre bundles of carbon nanotubes as electrochemical sensors for long-term in vivo monitoring of multiple disease biomarkers. Nat. Biomed. Eng. 2020, 4, 159–171.
- Kang, B.C.; Park, B.S.; Ha, T.J. Highly sensitive wearable glucose sensor systems based on functionalized single-wall carbon nanotubes with glucose oxidase-nafion composites. Appl. Surf. Sci. 2019, 470, 13–18.
- Yoo, E.H.; Lee, S.Y. Glucose biosensors: An overview of use in clinical practice. Sensors 2010, 10, 4558–4576.
- Gupta, S.; Murthy, C.N.; Prabha, C.R. Recent advances in carbon nanotube based electrochemical biosensors. Int. J. Biol. Macromol. 2018, 108, 687–703.
- Yang, M.H.; Jeong, S.W.; Chang, S.J.; Kim, K.H.; Jang, M.; Kim, C.H.; Bae, N.H.; Sim, G.S.; Kang, T.; Lee, S.J.; et al. Flexible and disposable sensing platforms based on newspaper. ACS Appl. Mater. Interfaces 2016, 8, 34978–34984.
- Yoon, J.; Lee, S.N.; Shin, M.K.; Kim, H.W.; Choi, H.K.; Lee, T.; Choi, J.W. Flexible electrochemical glucose biosensor based on GOx/gold/MoS2/gold nanofilm on the polymer electrode. Biosens. Bioelectron. 2019, 140, 83–89.
- Trieu, P.T.; Lee, N.Y. Paper-based all-in-one origami microdevice for nucleic acid amplification testing for rapid colorimetric identification of live cells for point-of-care testing. Anal. Chem. 2019, 91, 11013–11022.
- You, J.B.; Yoo, Y.; Oh, M.S.; Im, S.G. Simple and reliable method to incorporate the janus property onto arbitrary porous substrates. ACS Appl. Mater. Inter. 2014, 6, 4005–4010.
- Trinh, T.N.D.; La, H.C.; Lee, N.Y. Fully integrated and foldable microdevice encapsulated with agarose for long-term storage potential for point-of-care testing of multiplex foodborne pathogens. ACS Sens. 2019, 4, 2754–2762.
- Noh, J.Y.; Yoon, S.W.; Kim, Y.; Lo, T.V.; Ahn, M.J.; Jung, M.C.; Le, T.B.; Na, W.; Song, D.; Le, V.P.; et al. Pipetting-based immunoassay for point-of-care testing: Application for detection of the influenza A virus. Sci. Rep. 2019, 9, 16661.
- Kim, S.H.; Woo, H.C.; Kim, M.H. Solid-phase colorimetric sensing probe for bromide based on a tough hydrogel embedded with silver nanoprisms. Anal. Chim. Acta 2020, 1131, 80–89.
- Shaban, S.M.; Moon, B.S.; Kim, D.H. Selective and sensitive colorimetric detection of p-aminophenol in human urine and paracetamol drugs based on seed-mediated growth of silver nanoparticles. Environ. Technol. Innov. 2021, 22, 101517.
- Tran, V.K.; Gupta, P.K.; Park, Y.; Son, S.E.; Hur, W.; Lee, H.B.; Park, J.Y.; Kim, S.N.; Seong, G.H. Functionalized bimetallic IrPt alloy nanoparticles: Multi-enzyme mimics for colorimetric and fluorometric detection of hydrogen peroxide and glucose. J. Taiwan Inst. Chem. E 2021, 120, 336–343.
- Lee, J.I.; Jang, S.C.; Chung, J.; Choi, W.K.; Hong, C.; Ahn, G.R.; Kim, S.H.; Lee, B.Y.; Chung, W.J. Colorimetric allergenic fungal spore detection using peptide-modified gold nanoparticles. Sens. Actuators B Chem. 2021, 327, 128894.
- Han, G.R.; Koo, H.J.; Ki, H.; Kim, M.G. Paper/Soluble polymer hybrid-based lateral flow biosensing platform for high-performance point-of-care testing. ACS Appl. Mater. Interfaces 2020, 12, 34564–34575.
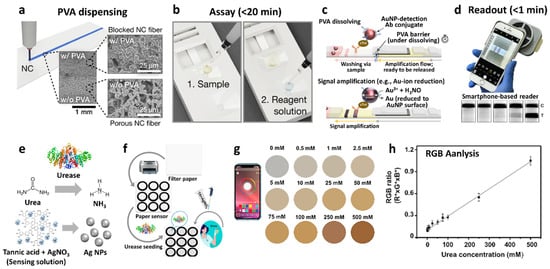
- Choi, C.K.; Shaban, S.M.; Moon, B.S.; Pyun, D.; Kim, D.H. Smartphone-assisted point-of-care colorimetric biosensor for the detection of urea via pH-mediated AgNPs growth. Anal. Chim. Acta 2021, 1170, 338630.
- Son, S.U.; Seo, S.B.; Jane, S.; Choi, J.; Lim, J.W.; Lee, D.K.; Kim, H.; Seo, S.; Kang, T.; Jung, J.; et al. Naked-eye detection of pandemic influenza a (pH1N1) virus by polydiacetylene (PDA)-based paper sensor as a point-of-care diagnostic platform. Sens. Actuators B Chem. 2019, 291, 257–265.
- Tawfik, S.M.; Elmasry, M.R.; Sharipov, M.; Azizov, S.; Lee, C.H.; Lee, Y.I. Dual emission nonionic molecular imprinting conjugated polythiophenes-based paper devices and their nanofibers for point-of-care biomarkers detection. Biosens. Bioelectron. 2020, 160, 112211.
- Han, K.N.; Choi, J.S.; Kwon, J. Three-dimensional paper-based slip device for one-step point-of-care testing. Sci. Rep. 2016, 6, 25710.
- Posthuma-Trumpie, G.A.; Korf, J.; van Amerongen, A. Lateral flow (immuno) assay: Its strengths, weaknesses, opportunities and threats. A literature survey. Anal. Bioanal. Chem. 2009, 393, 569–582.
- Li, F.; You, M.L.; Li, S.X.; Hu, J.; Liu, C.; Gong, Y.; Yang, H.Y.; Xu, F. Paper-based point-of-care immunoassays: Recent advances and emerging trends. Biotechnol. Adv. 2020, 3, 107442.
- Kim, K.; Kashefi-Kheyrabadi, L.; Joung, Y.; Kim, K.; Dang, H.J.; Chavan, S.G.; Lee, M.H.; Choo, J. Recent advances in sensitive surface-enhanced Raman scattering-based lateral flow assay platforms for point-of-care diagnostics of infectious diseases. Sens. Actuators B Chem. 2021, 329, 129214.
- Hang, G.R.; Ki, H.; Kim, M.G. Automated, universal, and mass-producible paper-based lateral flow biosensing platform for high-performance point-of-care testing. ACS Appl. Mater. Interfaces 2020, 12, 1885–1894.
- Kim, K.; Joung, H.A.; Han, G.R.; Kim, M.G. An immunochromatographic biosensor combined with a water-swellable polymer for automatic signal generation or amplification. Biosens. Bioelectron. 2016, 85, 422–428.
