The formulation of an ideal vaginal drug delivery system (DDS), with the requisite properties, with respect to safety, efficacy, patient compliance, aesthetics, harmonization with the regulatory requirements, and cost, requires a meticulous selection of the active ingredients and the excipients used. Novel excipients defined by diversity and multifunctionality are used in order to ameliorate drug delivery attributes. Synthetic and natural polymers are broadly used in pharmaceutical vaginal formulations (solid, semi-solid dosage forms, implantable devices, and nanomedicines) with a promising perspective in improving stability and compatibility issues when administered topically or systemically. Moreover, the use of biopolymers is aiming towards formulating novel bioactive, biocompatible, and biodegradable DDSs with a controllable drug release rate.
- novel vaginal excipients
- co-polymers
- mucoadhesive
- bio-adhesive
- thermosensitive
- vaginal drug delivery
- novel vaginal formulations
- vaginal films
- vaginal nanomedicines
- drug release
1. Introduction
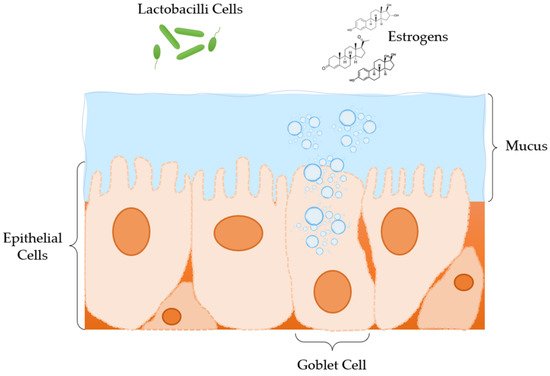
2. Anatomy and Physiology of the Vagina
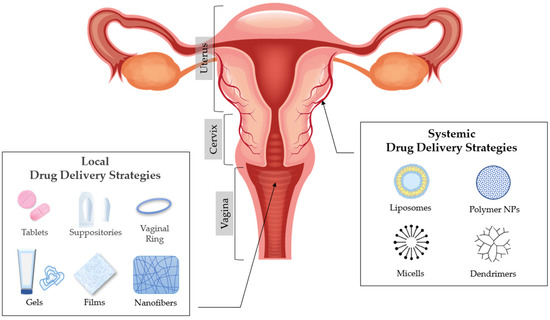
3. Vaginal Drug Delivery
4. Factors That Affect Drug Absorption in Vaginal Epithelium
5. Excipients Used in Modified Drug Release Pharmaceutical Dosage Forms for Vaginal Administration
5.1. Excipients Used in Matrix Tablets for Vaginal Administration
5.2. Excipients Used in Solid Dosage Forms for Vaginal Administration: Ovules and Vaginal Suppositories
5.3. Excipients Used in Semi-Solid Dosage Forms for Vaginal Administration: Gels
5.4. Excipients Used in Films for Vaginal Administration
5.6. Excipients Used in Nanomedicine for Vaginal Administration
6. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/ma15010327
References
- Kar, M.; Chourasiya, Y.; Maheshwari, R.; Tekade, R.K. Current Developments in Excipient Science: Implication of Quantitative Selection of Each Excipient in Product Development. In Advances in Pharmaceutical Product Development and Research, Basic Fundamentals of Drug Delivery; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 29–83.
- Alexander, N.J.; Baker, E.; Kaptein, M.; Karck, U.; Miller, L.; Zampaglione, E. Why consider vaginal drug administration? Fertil. Steril. 2004, 82, 1–12.
- Forsberg, J.G. A morphologist’s approach to the vagina. Acta Obstet. Gynecol. Scand. Suppl. 1996, 163, 3–10.
- Sjoberg, I. The vagina: Morphological, functional and ecological aspects. Acta Obstet. Gynecol. Scand. Suppl. 1992, 71, 84–85.
- Pendergrass, P.B.; Reeves, C.A.; Belovicz, M.W.; Molter, D.J.; White, J.H. The shape and dimensions of the human vagina as seen in three-dimensional vinyl polysiloxane casts. Gynecol. Obstet. Investig. 1996, 42, 178–182.
- Pendergrass, P.B.; Belovicz, M.W.; Reeves, C.A. Surface area of the human vagina as measured from vinyl polysiloxane casts. Gynecol. Obstet. Investig. 2003, 55, 110–113.
- Veiga, M.; Ruiz-Caro, R.; Mart, A. Polymer Gels in Vaginal Drug Delivery Systems. In Polymer Gels, Gels Horizons: From Science to Smart Materials; Thakur, V.K., Ed.; Springer: Singapore, 2018; pp. 197–246.
- Rohan, L.C.; Sassi, A.B. Vaginal drug delivery systems for HIV prevention. AAPS J. 2009, 11, 78–87.
- Walker, D.C.; Brown, B.H.; Blackett, A.D.; Tidy, J.; Smallwood, R.H. A study of the morphological parameters of cervical squamous epithelium. Physiol. Meas. 2003, 24, 121–135.
- Ferguson, L.M.; Rohan, L.C. The importance of the vaginal delivery route for antiretrovirals in HIV prevention. Ther. Deliv. 2011, 2, 1535–1550.
- Garg, S.; Vermani, K.; Kohli, G.; Kandarapu, R.; Tambwekar, K.R.; Garg, A.; Waller, D.P.; Zaneveld, L.J. Survey of vaginal formulations available on the Indian market: Physicochemical characterization of selected products. Int. J. Pharmaceut. Med. 2002, 16, 141–152.
- Vermani, K.; Garg, S. The scope and potential of vaginal drug delivery. Pharm. Sci. Technol. Today 2000, 3, 359–364.
- Garg, S.; Tambwekar, K.R.; Vermani, K.; Kandarapu, R.; Garg, A.; Waller, D.P.; Zaneveld, L.J. Development pharmaceutics of microbicide formulations. Part II: Formulation, evaluation, and challenges. AIDS Patient Care STDs 2003, 17, 377–399.
- Woolfson, A.D.; Malcolm, R.K.; Gallagher, R. Drug delivery by the intravaginal route. Crit. Rev. Ther. Drug Carr. Syst. 2000, 17, 509–555.
- Baloglu, E.; Senyigit, Z.A.; Karavana, S.Y.; Bernkop-Schnurch, A. Strategies to prolong the intravaginal residence time of drug delivery systems. J. Pharm. Pharm. Sci. 2009, 12, 312–336.
- Ozyazici, M.; Gökçe, E.; Hizarcioglu, S.Y.; Taner, M.S.; Köseoglu, K.; Ertan, G. Dissolution and vaginal absorption characteristics of metronidazole and ornidazole. Pharmazie 2006, 61, 855–861.
- Wong, T.W.; Dhanawat, M.; Rathbone, M.J. Vaginal drug delivery: Strategies and concerns in polymeric nanoparticle development. Expert Opin. Drug Deliv. 2014, 11, 1419–1434.
- Farage, M.; Maibach, H. Lifetime changes in the vulva and vagina. Arch. Gynecol. Obs. 2006, 273, 195–202.
- Burruano, B.T.; Schnaare, R.L.; Malamud, D. Synthetic cervical mucus formulation. Contraception 2002, 66, 137–140.
- Katz, D.F. Human cervical mucus: Research update. Am. J. Obstet. Gynecol. 1986, 165, 1984–1986.
- Zavos, P.M.; Cohen, M.R. The pH of cervical mucus and the postcoital test. Fertil. Steril. 1980, 34, 234–238.
- Buckheit, R.W.; Watson, K.M.; Morrow, K.M.; Ham, A.S. Development of topical microbicides to prevent the sexual transmission of HIV. Antivir. Res. 2010, 85, 142–158.
- Khanna, N.; Dalby, R.; Tan, M.; Arnold, S.; Stern, J.; Frazer, N. Phase I/II clinical safety studies of terameprocol vaginal ointment. Gynecol. Oncol. 2007, 107, 554–562.
- Khanna, N.; Dalby, R.; Connor, A.; Church, A.; Stern, J.; Frazer, N. Phase I clinical trial of repeat dose terameprocol vaginal ointment in healthy female volunteers. Sex. Transm. Dis. 2008, 35, 577–582.
- Burns, R.N.; Hendrix, C.W.; Chaturvedula, A. Population pharmacokinetics of tenofovir and tenofovir-diphosphate in healthy women. J. Clin. Pharmacol. 2015, 55, 629–638.
- Laeyendecker, O.; Redd, A.D.; Nason, M.; Longosz, A.F.; Karim, Q.A.; Naranbhai, V.; Garrett, N.; Eshleman, S.H.; Abdool Karim, S.S.; Quinn, T.C. Antibody maturation in women who acquire HIV infection while using antiretroviral preexposure prophylaxis. J. Infect. Dis. 2015, 212, 754–759.
- Marrazzo, J.M.; Ramjee, G.; Richardson, B.A.; Gomez, K.; Mgodi, N.; Nair, G.; Palanee, T.; Nakabiito, C.; van der Straten, A.; Noguchi, L.; et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N. Engl. J. Med. 2015, 372, 509–518.
- Clark, M.R.; Peet, M.M.; Davis, S.; Doncel, G.F.; Friend, D.R. Evaluation of rapidly disintegrating vaginal tablets of Tenofovir, Emtricitabine and their combination for HIV-1 prevention. Pharmaceutics 2014, 6, 616–631.
- Sánchez-Sánchez, M.P.; Martín-Illana, A.; Ruiz-Caro, R.; Bermejo, P.; Abad, M.J.; Carro, R.; Bedoya, L.M.; Tamayo, A.; Rubio, J.; Fernández-Ferreiro, A.; et al. Chitosan and Kappa-Carrageenan vaginal acyclovir formulations for prevention of genital herpes. In vitro and ex vivo evaluation. Mar. Drugs 2015, 13, 5976–5992.
- Melnyk, G.; Yarnykh, T.; Herasymova, I. Analytical review of the modern range of suppository bases. Syst. Rev. Pharm. 2020, 11, 503–508.
- Akil, A.; Agashe, H.; Dezzutti, C.S.; Moncla, B.J.; Hillier, S.L.; Devlin, B.; Shi, Y.; Uranker, K.; Rohan, L.C. Formulation and characterization of polymeric films containing combinations of antiretrovirals (ARVs) for HIV prevention. Pharm. Res. 2015, 32, 458–468.
- Johnson, T.J.; Gupta, K.M.; Fabian, J.; Albright, T.H.; Kiser, P.F. Segmented polyurethane intravaginal rings for the sustained combined delivery of antiretroviral agents dapivirine and tenofovir. Eur. J. Pharm. Sci. 2010, 39, 203–212.
- Smith, J.M.; Srinivasan, P.; Teller, R.S.; Lo, Y.; Dinh, C.T.; Kiser, P.F.; Herold, B.C. Tenofovir disoproxil fumarate intravaginal ring protects high-dose depot medroxyprogesterone acetate- treated macaques from multiple SHIV exposures. J. Acquir. Immune Defic. Syndr. 2015, 68, 1–5.
- Srinivasan, P.; Dinh, C.; Zhang, J.; Pau, C.P.; McNicholl, J.M.; Lo, Y.; Herold, B.C.; Teller, R.; Kiser, P.; Smith, J.M. Pharmacokinetic evaluation of tenofovir disoproxil fumarate released from an intravaginal ring in pigtailed macaques after 6 months of continuous use. J. Med. Primatol. 2014, 43, 364–369.
- Rehman, K.; Mohd Amin, M.C.; Zulfakar, M.H. Development and physical characterization of polymer-fish oil bigel (hydrogel/oleogel) system as a transdermal drug delivery vehicle. J. Oleo Sci. 2014, 63, 961–970.
- Sagiri, S.S.; Singh, V.K.; Kulanthaivel, S.; Banerjee, I.; Basak, P.; Battachrya, M.K.; Pal, K. Stearate organogel-gelatin hydrogel based bigels: Physicochemical, thermal, mechanical characterizations and in vitro drug delivery applications. J. Mech. Behav. Biomed. Mater. 2015, 43, 1–17.
- Singh, V.K.; Anis, A.; Banerjee, I.; Pramanik, K.; Bhattacharya, M.K.; Pal, K. Preparation and characterization of novel carbopol based bigels for topical delivery of metronidazole for the treatment of bacterial vaginosis. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 44, 151–158.
- Acarturk, F. Mucoadhesive Vaginal Drug Delivery Systems. Recent Pat. Drug Deliv. 2009, 3, 193–205.
