Functional ability is the basis of healthy aging. Articular cartilage degeneration is amongst the most prevalent degenerative conditions that cause adverse impacts on the quality of life; moreover, it represents a key predisposing factor to osteoarthritis (OA). Both the poor capacity of articular cartilage for self-repair and the unsatisfactory outcomes of available clinical interventions make innovative tissue engineering a promising therapeutic strategy for articular cartilage repair. Significant progress was made in this field; however, a marked heterogeneity in the applied biomaterials, biofabrication, and assessments is nowadays evident by the huge number of research studies published to date. Accordingly, this entry assimilates the most recent advances in cell-based and cell-free tissue engineering of articular cartilage and also focuses on the assessments performed via various in vitro studies, ex vivo models, preclinical in vivo animal models, and clinical studies in order to provide a broad overview of the latest findings and clinical translation in the context of degenerated articular cartilage and OA.
- cartilage regeneration
- tissue engineering
- biomaterials
1. Introduction
2. Age-Related Changes in Articular Cartilage
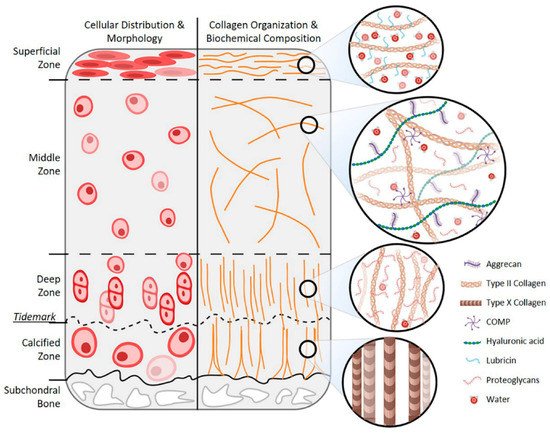
2.1. Age-Related Changes in Chondrocytes
2.2. Age-Related Changes in Cartilage ECM
3. Tissue Engineering Strategies for Articular Cartilage Regeneration
3.1. Cell-Based Tissue Engineering Strategies
3.1.1. Scaffold-Based Strategies
3.1.2. Scaffold-Free Strategies
3.1.3. Injectables
3.2. Cell-Free Tissue Engineering Strategies
3.2.1. Scaffold-Based Strategies
3.2.2. Injectables
This entry is adapted from the peer-reviewed paper 10.3390/ma15010031
References
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; d’Adda di Fagagna, F. Cellular Senescence in Ageing: From Mechanisms to Therapeutic Opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95.
- Baar, M.P.; Perdiguero, E.; Muñoz-Cánoves, P.; de Keizer, P.L. Musculoskeletal Senescence: A Moving Target Ready to Be Eliminated. Curr. Opin. Pharmacol. 2018, 40, 147–155.
- Lotz, M.; Loeser, R.F. Effects of Aging on Articular Cartilage Homeostasis. Bone 2012, 51, 241–248.
- Chang, L.-R.; Marston, G.; Martin, A. Anatomy, Cartilage; StatPearls Publishing: Treasure Island, FL, USA, 2020.
- Anderson, A.S.; Loeser, R.F. Why Is Osteoarthritis an Age-Related Disease? Best Pract. Res. Clin. Rheumatol. 2010, 24, 15–26.
- Cieza, A.; Causey, K.; Kamenov, K.; Hanson, S.W.; Chatterji, S.; Vos, T. Global Estimates of the Need for Rehabilitation Based on the Global Burden of Disease Study 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2021, 396, 2006–2017.
- Medvedeva, E.V.; Grebenik, E.A.; Gornostaeva, S.N.; Telpuhov, V.I.; Lychagin, A.V.; Timashev, P.S.; Chagin, A.S. Repair of Damaged Articular Cartilage: Current Approaches and Future Directions. Int. J. Mol. Sci. 2018, 19, 2366.
- Pogliacomi, F.; Schiavi, P.; Paraskevopoulos, A.; Leigheb, M.; Pedrazzini, A.; Ceccarelli, F.; Vaienti, E. When Is Indicated Viscosupplementation in Hip Osteoarthritis? Acta Biomed. 2018, 90, 67–74.
- Leigheb, M.; Bosetti, M.; de Consoli, A.; Borrone, A.; Cannas, M.; Grassi, F. Chondral Tissue Engineering of the Reumatoid Knee with Collagen Matrix Autologous Chondrocytes Implant. Acta Biomed. 2017, 88, 107–113.
- Moreira-Teixeira, L.S.; Georgi, N.; Leijten, J.; Wu, L.; Karperien, M. Cartilage Tissue Engineering. Endocr. Dev. 2011, 21, 102–115.
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sports Health 2009, 1, 461–468.
- Thorp, H.; Kim, K.; Kondo, M.; Maak, T.; Grainger, D.W.; Okano, T. Trends in Articular Cartilage Tissue Engineering: 3D Mesenchymal Stem Cell Sheets as Candidates for Engineered Hyaline-like Cartilage. Cells 2021, 10, 643.
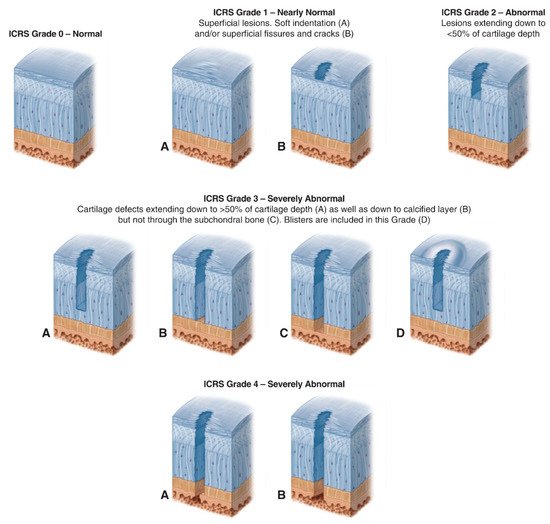
- International Cartilage Repair Society (ICRS) Cartilage Injury Evaluation Package. Available online: https://cartilage.org/content/uploads/2014/10/ICRS_evaluation.pdf (accessed on 18 October 2021).
- Kwon, H.; Brown, W.E.; Lee, C.A.; Wang, D.; Paschos, N.; Hu, J.C.; Athanasiou, K.A. Surgical and Tissue Engineering Strategies for Articular Cartilage and Meniscus Repair. Nat. Rev. Rheumatol. 2019, 15, 550–570.
- Zhao, X.; Hu, D.A.; Wu, D.; He, F.; Wang, H.; Huang, L.; Shi, D.; Liu, Q.; Ni, N.; Pakvasa, M.; et al. Applications of Biocompatible Scaffold Materials in Stem Cell-Based Cartilage Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 603444.
- Johnson, K.; Zhu, S.; Tremblay, M.S.; Payette, J.N.; Wang, J.; Bouchez, L.C.; Meeusen, S.; Althage, A.; Cho, C.Y.; Wu, X.; et al. A Stem Cell-Based Approach to Cartilage Repair. Science 2012, 336, 717–721.
- Cochis, A.; Bonetti, L.; Sorrentino, R.; Negrini, N.C.; Grassi, F.; Leigheb, M.; Rimondini, L.; Farè, S. 3D Printing of Thermo-Responsive Methylcellulose Hydrogels for Cell-Sheet Engineering. Materials 2018, 11, 579.
- Altomare, L.; Cochis, A.; Carletta, A.; Rimondini, L.; Farè, S. Thermo-Responsive Methylcellulose Hydrogels as Temporary Substrate for Cell Sheet Biofabrication. J. Mater. Sci. Mater. Med. 2016, 27, 95.
- Wasai, S.; Toyoda, E.; Takahashi, T.; Maehara, M.; Okada, E.; Uchiyama, R.; Akamatsu, T.; Watanabe, M.; Sato, M. Development of Injectable Polydactyly-Derived Chondrocyte Sheets. Int. J. Mol. Sci. 2021, 22, 3198.
- Cochis, A.; Grad, S.; Stoddart, M.J.; Farè, S.; Altomare, L.; Azzimonti, B.; Alini, M.; Rimondini, L. Bioreactor Mechanically Guided 3D Mesenchymal Stem Cell Chondrogenesis Using a Biocompatible Novel Thermo-Reversible Methylcellulose-Based Hydrogel. Sci. Rep. 2017, 7, 45018.
- Szychlinska, M.A.; Calabrese, G.; Ravalli, S.; Dolcimascolo, A.; Castrogiovanni, P.; Fabbi, C.; Puglisi, C.; Lauretta, G.; di Rosa, M.; Castorina, A.; et al. Evaluation of a Cell-Free Collagen Type I-Based Scaffold for Articular Cartilage Regeneration in an Orthotopic Rat Model. Materials 2020, 13, 2369.
- Yuan, T.; Li, Z.; Zhang, Y.; Shen, K.; Zhang, X.; Xie, R.; Liu, F.; Fan, W. Injectable Ultrasonication-Induced Silk Fibroin Hydrogel for Cartilage Repair and Regeneration. Tissue Eng. Part A 2021, 27, 1213–1224.
