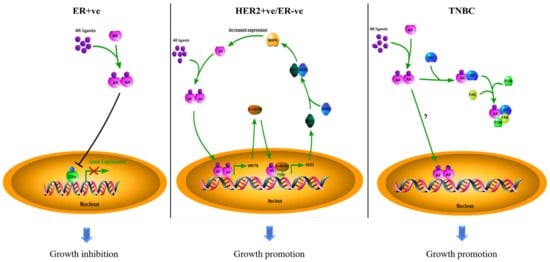
Biomarkers can be used for diagnosis, prognosis, and prediction in targeted therapy. The estrogen receptor α (ERα) and human epidermal growth factor receptor 2 (HER2) are standard biomarkers used in breast cancer for guiding disease treatment. The androgen receptor (AR), a nuclear hormone receptor, contributes to the development and progression of prostate tumors and other cancers. With increasing evidence to support that AR plays an essential role in breast cancer, AR has been considered a useful biomarker in breast cancer, depending on the context of breast cancer sub-types. The existing survival analyses suggest that AR acts as a tumor suppressor in ER + ve breast cancers, serving as a favorable prognostic marker. However, AR functions as a tumor promoter in ER-ve breast cancers, including HER2 + ve and triple-negative (TNBC) breast cancers, serving as a poor prognostic factor. AR has also been shown to be predictive of the potential of response to adjuvant hormonal therapy in ER + ve breast cancers and to neoadjuvant chemotherapy in TNBC.
All contents are adapted from You, C.-P.; Leung, M.-H.; Tsang, W.-C.; Khoo, U.-S.; Tsoi, H. Androgen Receptor as an Emerging Feasible Biomarker for Breast Cancer. Biomolecules 2022, 12, 72. https://doi.org/10.3390/biom12010072
- breast cancer
- androgen receptor
- biomarker
- targeted therapy
1. What Are Cancer Biomarkers
2. AR as a Biomarker in Breast Cancers
| Types | AR Status (Cut-Off Used to Define AR + ve) | Case No. | Indicator of Clinical Outcomes 1 | Hazard Ratio (HR) | 95% Confidence Interval (CI) | p-Value | Reference |
|---|---|---|---|---|---|---|---|
| ER + ve | Positive (≥10% nuclear-stained) | 470 | DFS | 0.654 | 0.429–0.997 | 0.049 | [44] |
| Negative (<10% nuclear-stained) | 202 | 1 | - | - | |||
| Positive (≥1% nuclear-stained) | 1024 | OS | 0.68 | 0.52–0.88 | - | [45] | |
| Negative (<1% nuclear-stained) | 140 | 1 | - | - | |||
| Positive (≥1% nuclear-stained) | 2833 | BCM | 0.53 | 0.41 –0.69 | < 0.001 | [46] | |
| Negative (<1% nuclear-stained) | 470 | 1 | - | - | |||
| Positive (≥1% nuclear-stained) | 609 | DSS | 0.259 | 0.139–0.482 | 0.000 | [47] | |
| Negative (<1% nuclear-stained) | 250 | 1 | - | - | |||
| High (mRNA Z-score) | 145 | DRFS | - | - | 0.008 | [48] | |
| Low (mRNA Z-score) | 144 | - | - | - | |||
| Positive (N/A) | - | DFS | 0.40 | 0.31–0.52 | < 0.001 | [54] | |
| Negative (N/A) | - | 1 | - | - | |||
| Positive (≥10% nuclear-stained) | 909 | OS | 0.71 | 0.53–0.95 | 0.022 | [55] | |
| Negative (<10% nuclear-stained) | 162 | 1 | - | - | |||
| Positive (≥1% nuclear-stained) | 461 | DFS | 0.606 | 0.388–0.944 | 0.027 | [56] | |
| Negative (<1% nuclear-stained) | 337 | 1 | - | - | |||
| HER2 + ve/ ER-ve |
Positive (≥10% nuclear-stained) | 49 | OS | - | - | 0.074 | [44] |
| Negative (<10% nuclear-stained) | 42 | - | - | - | |||
| High (mRNA level) | 35 | DFS | 1.46 | 1.03–2.06 | 0.03 | [57] | |
| Low (mRNA level) | 49 | 1 | - | - | |||
| TNBC | Positive (≥1% nuclear-stained) | 78 | OS | 1.83 | 1.11–3.01 | 0.02 | [45] |
| Negative (<1% nuclear-stained) | 133 | 1 | - | - | |||
| Positive (≥1% nuclear-stained) | 261 | OS | 2.159 | 1.224–3.808 | 0.008 | [58] | |
| Negative (<1% nuclear-stained) | 231 | 1 | - | - | |||
| Positive (≥1% nuclear-stained) | 23 | DFS | 5.26 | 1.39–19.86 | 0.014 | [59] | |
| Negative (<1% nuclear-stained) | 38 | 1 | - | - | |||
| Positive (≥1% nuclear-stained) | 78 | DDFS | 1.82 | 1.10–3.02 | 0.020 | [60] | |
| Negative (<1% nuclear-stained) | 185 | 1 | - | - |
2.1. The Role of AR in ER + ve Breast Cancer
2.2. The Role of AR in HER2 + ve Breast Cancer
2.3. The Role of AR in TNBC
2.4. Conflicting Results
This entry is adapted from the peer-reviewed paper 10.3390/biom12010072
